1. Water homeostasis and cell volume maintenance & regulation
We are like little bags of sea water walking the surface of the earth...
As a terrestrial species, we carry water away from streams, rivers, ponds, and seas. As water flows through our bodies,
we maintain a condition of steady-state. We constantly intake water through drinks and food and loose water through
respiration, sweat, urine, and feces. The volume of water we circulate in our heart, arteries and veins define our blood
pressure. The amount of water in cells (which define cell volume) is also maintained constant and regulated when
disrupted. This is essential for the proper architecture and function of tissues and organs.The laboratory studies the
mechanisms of water homeostasis.
2. Chloride homeostasis in central and peripheral neurons
GABAergic and glycinergic synaptic transmission depends upon the Cl- gradient that exists across the neuronal
membrane. The neuronal Cl- concentration is regulated by electroneutral cation-chloride cotransporters such as
NKCC1 (Na-K-2Cl cotransporter) and KCC2 (K-Cl cotransporter). In mature CNS neurons, KCC2 drives Cl- to
concentrations below thermodynamical potential equilibrium strengthening the driving force for GABA-mediated
Cl- influx and membrane hyperpolarization. In PNS neurons, NKCC1 drives Cl- to concentrations well above
thermodynamical potential equilibrium leading to membrane depolarization and pre-synaptic inhibition.
Role of KCC2 in hyperexcitability and epilepsy.
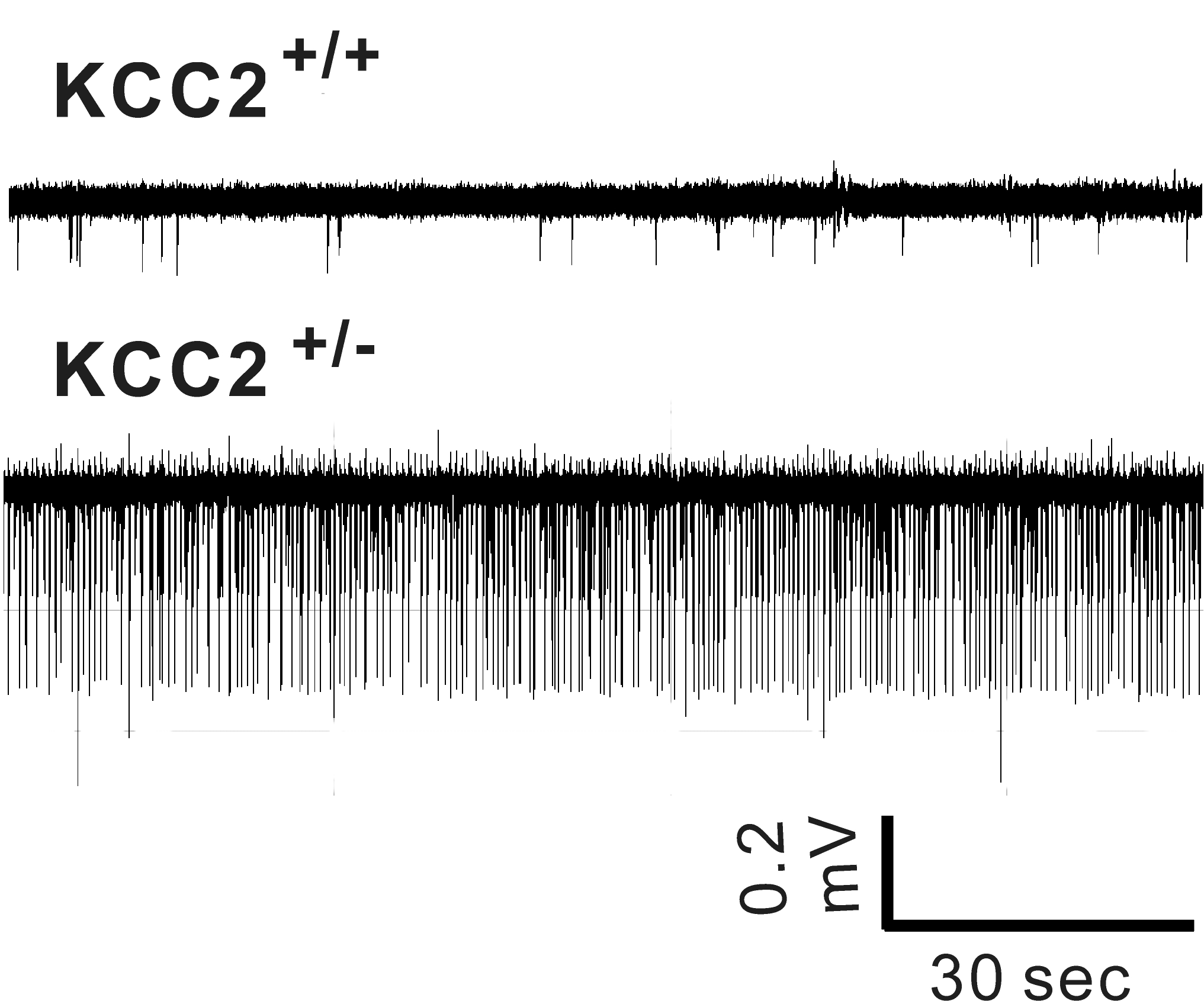 Hyperexcitability in CA1 hippocampus of KCC2+/- (heterozygote) mice
Hyperexcitability in CA1 hippocampus of KCC2+/- (heterozygote) mice
In rodents, KCC2 expression is low at birth and increases during postnatal development. This increase parallels
a decrease in intracellular Cl- and the switch from GABA depolarization to hyperpolarization. Reduction in KCC2 expression and activity lead to
hyperexcitability. The laboratory created a KCC2b knockout mouse reducing overall KCC2 expression by 90%. The animals die of seizures before
weaning. As shown in the attached Figure, mice with 50% KCC2 expression (heterozygote mice) demonstrate
increased excitability compared to
wild-type mice. The laboratory studies the properties, pharmacology, and regulation of the cotransporter.
Role of KCC3 in peripheral nerve physiology and pathology.
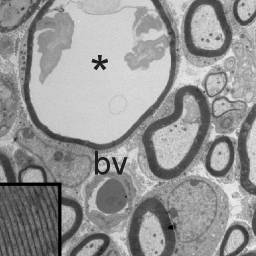
Abnormal periaxonal fluid accumulation in knockout KCC3 mice
Mutations in SLC12A6, the gene encoding KCC3, result in a peripheral neuropathy disorder called ACCPN (Agenesis of
Corpus Callosum with Peripheral Neuropathy). The laboratory uses heterologous expression systems to study the function
of the cotransporter KCC3 (dimerization, phosphorylation, etc.) and genetically-modified mouse models to study the
degeneration of peripheral nerves. We have found in our KCC3 knockout model that juvenile mice develop periaxonal
swelling in sciatic nerves prior to adult neurodegeneration. Current and future research projects are focusing on the
regulation of KCC3 and its role in Cl- homeostasis and cell volume maintenance and regulation.
3. Control of Na+ reabsorption in the distal nephron
Most of the Na+ which is filtered by the kidney glomeruli is reabsorbed by the renal tubule. While the vast majority is
reabsorbed in the proximal tubule, the regulated portion is reabsorbed in the distal nephron through the Na-K-2Cl
cotransporter NKCC2 in the Thick Ascending Limb of Henle (TAL), the Na-Cl cotransporter NCC in the distal
convoluted tubule, and the epithelial Na+ channel ENac in the collecting duct. The laboratory studies the mechanisms
(kinases) regulating Na+ reabsorption in the TAL and DCT.
4. Basic mechanisms of ion transport
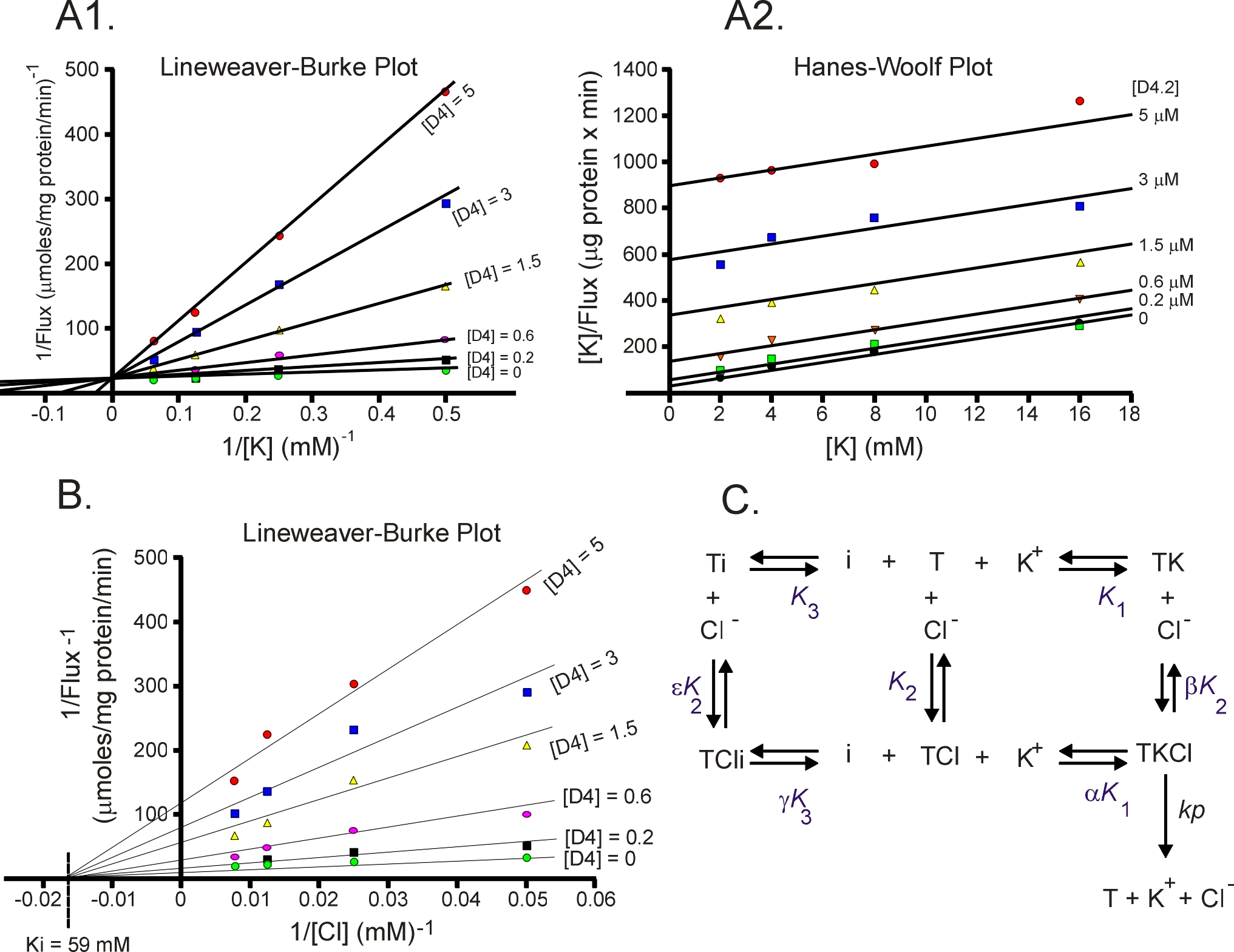 Enzyme kinetics, the steady-state approach
Enzyme kinetics, the steady-state approach
The laboratory studies the molecular properties of ion transporters. This includes structure - activity relationship,
transport kinetics, transport regulation, etc. The attached Figure was extracted from a study that identified novel
KCC2 inhibitors (PNAS 106: 5383-5388, 2009) . Using steady-state kinetics, we determined that the inhibitor D2
(now named ML077) binds competitively with K+ but non-competitively with Cl-. This is demonstrated in panel A
by a unique intercept of the lines on the Y axis (Lineweaver-Burke plot) as D4 and K+ are varied whereas different
intercepts on the Y axis (panel B) as D4 and Cl- are varied. These data indicate that the inhibitor must bind the
transporter before K+. Once K+ is bound, the inhibitor cannot bind.