Research Projects
-
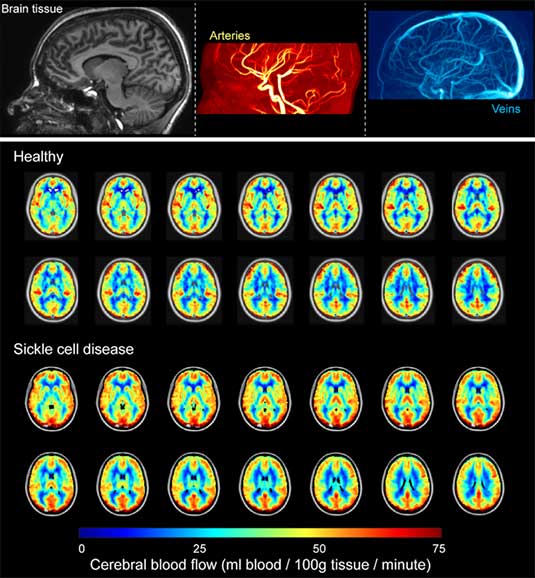
The overall goal of this work is to expand our understanding of the basic pathophysiology of neurological morbidity in anemia using novel MRI-derived measures of oxygen extraction fraction (OEF). Sickle cell anemia (SCA) is a well-characterized monogenetic disorder with an abnormal form of hemoglobin (Hb) S that leads to many complications including a high prevalence of cerebral vasculopathy, silent cerebral infarcts, and overt strokes, 100-fold greater than other children. Secondary prevention of recurrent cerebral infarcts in children with SCA includes monthly blood transfusion therapy indefinitely. This strategy significantly reduces the risk of recurrent overt and silent strokes bu with substantial transfusion-related morbidity. Many children must be transfused to prevent one recurrent infarct. Improved abilities are required to identify and stratify children at the highest risk of stroke. The critical barrier rests with a general inability to identify underlying brain tisue-level impairment that may provide evidence-based biomarkers for stroke risk. Hemodynamic failure, measured by increased OEF (the ratio of oxygen consumed to oxygen delivered) in the brain, is associated with increased stroke risk. Limited widespread availability of methodologies for measuring OEF has prevented evaluation of OEF rigorous clinical studies in children with SCA at risk of infarct recurrence. Our team has recently demonstrated an ability to utilize MRI to measure OEF noninvasively. We are working to apply this method in children with SCA to test fundamental hypotheses about the relationships between OEF, other hemodynamic factors such as cerebral blood flow, and cerebral infarcts.
-
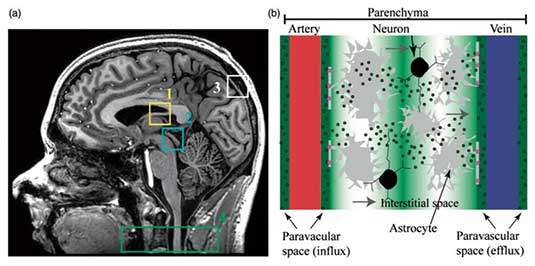
Immunological and physiological studies have recently provided strong evidence in support of a central nervous system (CNS) lymphatic drainage system in vertebrate animals, which has more recently been implicated in amyloid beta (Aβ) plaque clearance disorders such as Alzheimer's disease (AD)-related dementias (ADRD). This system comprises (i) dural and meningeal lymphatic vessels that drain CSF and interstitial fluid (ISF) toward cervical lymph nodes and which may (ii) communicate with the recently-proposed glymphatic system, an aquaporin-4 (AQP4)-mediated system that facilitates CSF-ISF efflux from periarterial to perivenous spaces and ultimately to cervical lymphatic vessels and nodes. While multiple independent studies have speculated that the CNS lymphatic system may have relevance to clearance conditions of unknown etiology (including but not limited to Aβ clearance deficiency and Alzheimer's disease), limited direct information is available on the relevance of this system to CNS clearance disorders in humans. The critical barrier to addressing this problem in patients with these conditions rests with a general lack of imaging methods that can be applied to interrogate multiple aspects of the proposed human CNS lymphatic system in vivo. The parent study focuses on developing magnetic resonance imaging (MRI) methodologies to visualize lymphatic dysfunction in the periphery of patients with known lymphatic dysfunction. Very recently, we have translated these noninvasive MRI methods, optimized in prior work to evaluate peripheral blood and lymphatic circulatory dysfunction, to the CNS. These methods provide a foundation whereby novel, noninvasive metrics can be measured and quantified to understand CNS lymphatic function in healthy tissue and also in the presence of increased Aβ burden and ADRD. As such, the goal of this work is to apply novel MRI and established PET approaches, optimized for evaluating peripheral circulatory dysfunction and Aβ deposition, respectively, to evaluate relationships between CNS glymphatic function, Aβ burden, and cognitive impairment in older adults with and at risk for Alzheimer's disease. Study findings are intended to provide imaging biomarkers of CNS lymphatic dysfunction which can be recorded noninvasively in vivo using clinically-available imaging equipment. If successful, results will provide a new avenue for both understanding AD pathophysiology and evaluating novel glymphatic-based therapeutic avenues in patients with ADRD.
-
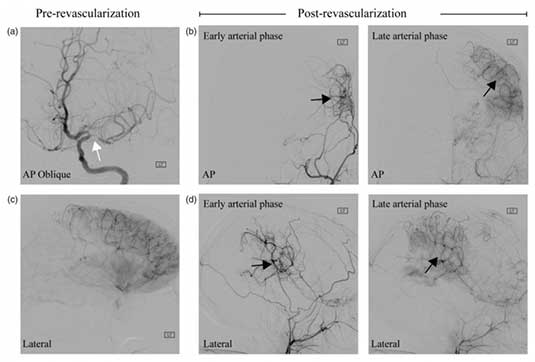
The goal of this work is to apply novel neuroimaging methods in patients with Moyamoya syndrome to test fundamental hypotheses regarding hemodynamic compensation, stroke history, and symptomatology. Moyamoya disease (MMD) has unknown etiology and is characterized by steno-occlusion of the supraclinoid internal carotid arteries and proximal branches, development of collateral vessels, and a high risk of stroke. Idiopathic MMD is relatively rare, however moyamoya syndrome (MMS), which can arise secondary to Down syndrome, sickle cell disease, atherosclerosis, and radiotherapy shares many phenotypical characteristics as idiopathic MMD, yet is observed much more frequently. Patients with MMS are at high risk for stroke, and compared with atherosclerotic disease where preferred treatment regimens are outlined by recent clinical trial results, optimal MMS therapies are less clear and may comprise medical management and/or surgical revascularization. Owing to the dynamic course of MMS, which includes a wide variation of progressive steno- occlusion, abnormal expression of endothelial growth factors and inflammatory proteins, hemo-metabolic disturbances, intimal vessel wall thickening, and neoangiogensis, there is a pressing clinical need to understand the pathophysiology of these processes, how they relate to symptomatology and stroke incidence, and ultimately may be used to stratify patients for therapy or guide development of novel pharmaceuticals. The critical barrier to achieving this rests with a lack of optimal methods that can be implemented for mapping and surveillance. Here, in adults and children with MMS, we implement new MRI methods developed in our center to test focused hypotheses regarding relationships between endothelial dysfunction, stroke incidence, and symptomatology; changes in vessel wall morphology, disease chronicity, and neurological symptoms; and oxygen extraction fraction response to surgical revascularization therapy. The short-term significance of this work is that it will improve our understanding of the physiological processes that underlie how tissue responds to proximal non-atherosclerotic steno-occlusion and revascularization, which will serve as a prerequisite for utilizing functional neuroimaging to stratify patients with MMS for appropriate therapy. The longer-term goal is to use this information to guide the development of novel pharmaceuticals or early screening procedures that may enable therapies to be titrated to patients prior to irreversible tissue damage.
-
Dopamine medications are effective in treating the motor symptoms of Parkinson’s Disease (PD), but dopamine agonists can trigger impulsive-compulsive behaviors (ICBs), such as compulsive gambling, eating or shopping, in a subset of patients. ICBs are thought to be caused by overstimulation of the mesocorticolimbic dopamine network, which regulates reward learning and executive function.
In a collaborative effort with Manus Donahue Ph.D., Daniel Claassen, M.D., and colleagues explored the neural underpinnings of ICBs in PD using a noninvasive imaging technique called arterial-spin-labeling (ASL)-MRI. ASL-MRI quantitatively measures cerebral blood flow (CBF), an indirect measure of brain metabolism and activity.
Comparing PD patients with and without ICBs, the researchers found that dopamine agonists increase CBF in brain regions of the mesocorticolimbic network only in patients with ICBs. They also found a link between dopamine agonist-induced changes in the mesocorticolimbic network and the expression of ICBs as well as their severity across all PD patients.
This study, published in Movement Disorders, highlights the potential of using ASL-MRI to predict ICB susceptibility in patients and improve clinical treatment plans.
This research was supported by grants from the National Institute of Neurological Disorders and Stroke (NS097783, NS080988), the American Heart Association, and an award from the National Center for Advancing Translational Sciences (TR000445).
-
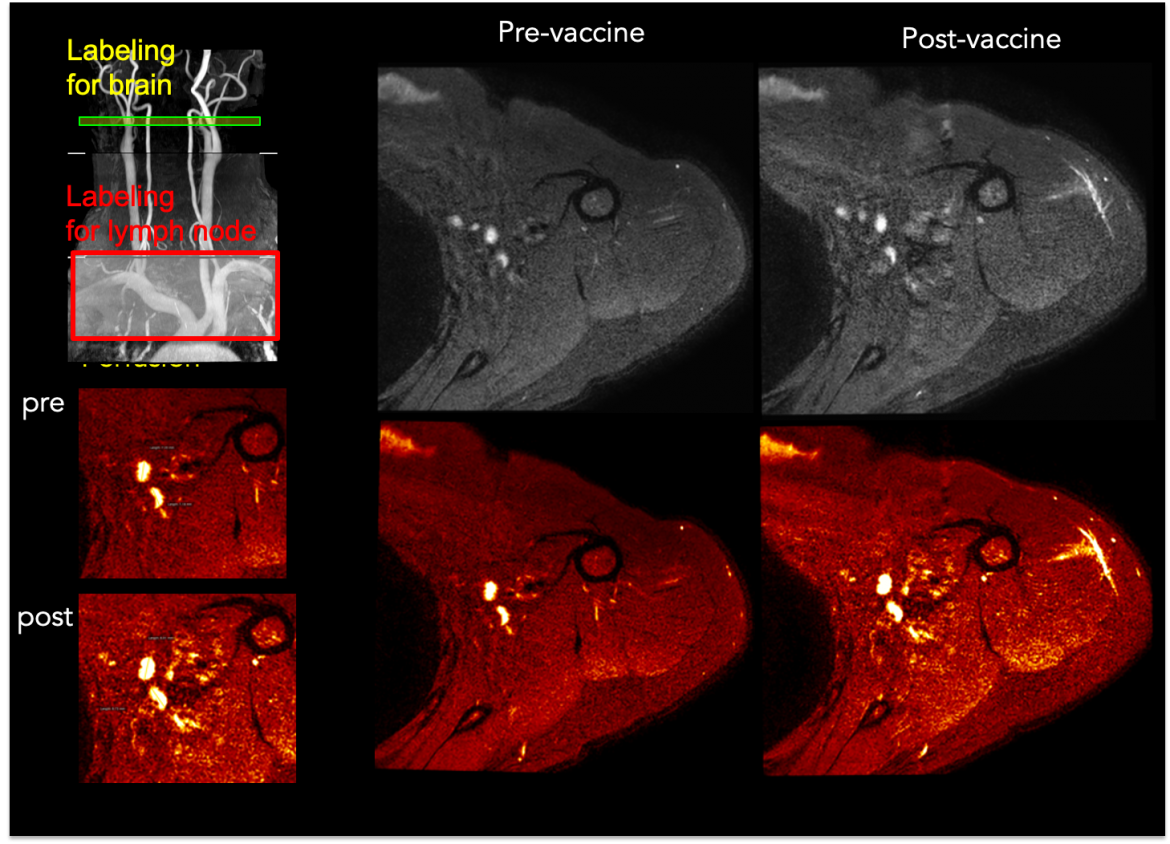 This line of work is focused on lymphatic markers of viral persistence in both healthy adults as well as adults with human immunodeficiency virus (HIV).
This line of work is focused on lymphatic markers of viral persistence in both healthy adults as well as adults with human immunodeficiency virus (HIV).Even in the presence of standard-of-care combination antiretroviral therapy (ART), HIV persists through viral reservoirs, which are comprised of infected, latent cells not actively producing HIV. These cells have reactivation potential, however identifying the location and extent of these viral reservoirs has remained elusive using conventional assays. Reservoirs often localize within secondary lymphoid tissues such as lymph nodes (LNs), yet lymphoid tissues are widespread and often difficult to access without LN biopsy or aspiration, and imaging approaches for visualizing lymphatic function remain underdeveloped.
HIV production during ART is associated with lymphatic hyperactivity, higher T cell markers of inflammation, and local inflammatory environments are likely fundamental to providing target cells for HIV spread and blunting adaptive immune responses. As such, it is logical that hemo-metabolic markers of LN activity, as well as lymphatic pumping kinetics, could provide information on local inflammation and in turn viral reservoirs. Very recently, it has been shown that LN activity asassessed by fluorodeoxyglucose (FDG) PET is higher in HIV-infected individuals than controls, and also that the level of LNactivity is associated with markers of viral persistence and T-cell activation. These data suggest that elevated LN metabolismmay provide an indicator of viral reservoirs.
The significance of assessing the topography and extent of viral reservoirs in vivo in humans is fundamental to the development of therapies that aim to eradicate or suppress reactivation. These approaches could focus on oral pharmaceuticals, reinfusion of host cells, or antibody therapies, however triaging patients for these promising therapies will require quantitative markers of viral reservoirs, ideally which can be assessed over a large spatial range and without exogenous contrast agents that may otherwise be contraindicated for longitudinal trials or surveillance. In this work, we directly address this problem by applying non-invasive MRI methods, sensitive to lymphatic function, in HIV patients with varying viral load. Current work is focused on quantifying the hemodynamic and molecular response of axillary LNs to vaccines and comparing these metrics with circulating pTh help B cells.
-
This work is intended to explore the mechanisms underlying the glymphatic system by complementary and integrative health approaches.Specifically, mindfulness meditation (MM) and associated contemplative exercises are fundamental to the mental health of a growing fraction of the population, however these practices are not widely accepted by all medical practitioners owing to a lack of objective, mechanistic evidence of how these therapies impact cerebral physiology. This issue is fundamental, as MM will not be widely accepted as a therapeutic tool until symptomatic changes are substantiated by quantitative, mechanistic descriptors. MM is hypothesized to be grounded in similar physiological processes as occur during sleep. Recent elegant work has demonstrated the role of a glymphatic waste clearance system, which is primarily active during sleep and serves to clear cerebral waste products. In this system, cerebrospinal fluid (CSF) and interstitial fluid efflux from periarterial to perivenous spaces acts in communication with potential meningeal and dural lymphatic channels to clear protein and waste products to cervical lymph nodes. Dysfunction of this system has recently been hypothesized to underlie a range of neurodegenerative clearance disorders with unknown etiology, including but not limited to Alzheimer’s disease (AD), Parkinson’s disease (PD), and multiple sclerosis. However, glymphatic function remains incompletely characterized in humans, largely due to a lack of safe methods for quantifying system components routinely and across different behavioral states. Our work has focused on developing non-invasive magnetic resonance imaging methods to quantify (i) perivascular diffusion, indicative of glymphatic currents; (ii) cerebral and peripheral tissue hemodynamics and function; and (iii) bulk CSF flow velocity. These methods were first applied and validated in individuals with peripheral lymphatic or cerebrovascular dysfunction of known etiology and are now being used to evaluate how glymphatic and associated perivascular flow dysfunction may mediate neurodegenerative pathophysiology in individuals with PD or AD. Here, we propose to extend our ongoing studies of glymphatic dysfunction in individuals with neurodegeneration to test fundamental hypotheses regarding the role of bulk and perivascular CSF flow to Aim (1) quantify perivascular diffusion, indicative of glymphatic currents, during sleep and wakefulness; to quantify similarities between these measures of glymphatic flow Aim (2) for sleep and open awareness MM, and Aim (3) before and after controlled MM training in older adults with and without PD-related neurodegeneration. Study findings are intended to inform the relevance of the traditional and well-characterized bulk CSF flow pathway, as well as more novel glymphatic pathway, on cerebral health during and following MM training. Study findings should inform the mechanism by which MM contributes to cognitive changes during active MM, as well as the potential of MM to be used as a cognitive therapy in health and neurodegeneration.