CONGRATULATIONS!

Tanya Stellges, CRCST
Program Director, Patient Safety, High-Level Disinfection & Sterilization
VUMC Credo Award Winner

VUMC Elevate Team Award Winners - Feb 2021
As part of Vanderbilt's mission to create safe patient care through education, research, and quality care, the department of Infection Prevention provides evidence-based, scientific, and proven resources to Vanderbilt faculty and staff as well as our patients and families. Through surveillance activities we detect potential healthcare-associated infections and develop action plans in collaboration with our quality and unit-based partners to mitigate those risks. We are a liaison to the Tennessee Department of Health and report communicable diseases for the institution as well as establishing a link to public health infrastructure in an effort to provide quality care to our patients. We are the content experts for infection prevention operations, research and educational activities, a position that we exercise regularly through our numerous publications and abstract presentations both inside and outside of the institution.
Please use the navigation at the top to learn more about our department's functions and activities.
-
MEASLES FAQ (CLICK for ACCESS)
Epidemiology: Cases are infectious four days before rash onset through four days after rash onset. Average incubation period is 14 days (range 7-21 days) between exposure and rash onset. Measles is transmitted via respiratory droplets and is highly infectious. The virus may linger in the air of a room for up to two hours after an infectious person has left the area.
Clinical Symptoms: Begins with a prodrome of fever (up to 105°F) and malaise, cough, conjunctivitis, and runny nose (coryza). Small bluish-white spots with red bases may be seen on the buccal mucosa (Koplik’s spots). Rash onset is typically 3-7 days after onset of the prodromal symptoms, beginning on the face and spreading downward. Complications may include bacterial superinfections or encephalitis. Consider the possibility of measles when evaluating susceptible patients with an acute febrile rash illness.
***Tennessee Department of Health Measles Diagnosis Algorithm (UPDATED MAY 7, 2019)***
Prevention: Vaccination is extremely effective, with two doses of MMR vaccine providing immunity to >97% of recipients. Ensure all patients ages 12 months and older are appropriately immunized and administer measles-mumps-rubella (MMR) vaccine to anyone over age 12 months who has not received MMR vaccine in the past 28 days and who does not have documentation of having received 2 doses of the vaccine. Infants may receive MMR vaccine after age 6 month in the event of an outbreak; however, the child would still require two doses after the first birthday to be considered appropriately vaccinated.
All VUMC healthcare providers should
- Consider measles in patients with the acute onset of symptoms of measles fever (up to 105°F) and malaise, runny nose (coryza), cough, conjunctivitis. Small white spots on a red base may be seen on the buccal mucosa (Koplik’s spots). A macular rash appears 3-7 days after the onset of prodromal symptoms, beginning on the face and spreading downward. Consider measles when evaluating patients with an acute febrile rash illness.
- Mask the patient and place these patients in Airborne Precautions in a negative pressure room with the door shut. For clinics with a suspected case, place a surgical mask on the patient at the point of entry (i.e. greeter's desk, reception desk). Do not put patient in the waiting room. Immediately bring patient to a room. Please place patient in a room, leave mask on the patient, and close the door. Staff entering the room wear an N-95 respirator.
- Notify Infection Prevention immediately using the on call pager 615-835-1205.
- IP and the Hospital Epidemiologist will notify the Tennessee Department of Health.
- Blood specimen (red top tube) and a throat swab (on viral transport medium) is collected from the patient and sent to the state lab. IP facilitates through the VUMC Laboratory.
- Prevention is essential with documented 2 doses of MMR vaccine especially for healthcare personnel.
- Healthcare personnel must have received two doses of MMR 28 days apart.
- Birth before 1957 DOES NOT qualify for presumptive evidence of immunity in a community with an ongoing outbreak.
- Resources for the public and medical community are available at: https://www.cdc.gov/measles/. For questions or additional information, please contact the VUMC Department of Infection Prevention via pager 615-835-1205.
Resources:
CDC website at https://www.cdc.gov/measles/hcp/index.html
Measles outbreaks or healthcare facilities: http://www.cdc.gov/vaccines/pubs/surv-manual/chpt07-measles.pdf
Tennessee Department of Health Measles Diagnosis Algorithm
VUMC Lab Forms:
-
An important intervention to reduce the risk of Staphylococcal infections (including MRSA and line infections), we have been using povidone iodine (PI) for nasal decolonization for several years. Feedback from our frontline providers helped illustrate some key barriers to the use of the PI, including difficulty tracking the number of completed doses, lack of clarity on the intervention benefit, and patient refusal. To help reduce these barriers, we will be moving to a new product for nasal decolonization, mupirocin. Some key points to know:
- Use of mupirocin, as an antibiotic, requires a provider order. We have worked with IT to link the nasal decolonization panel into existing ICU admission order sets. In addition, starting July 18, 2024, an new requirement for an order to allow access of a central venous catheter or midline will take effect. This new order will include the nasal decolonization panel.
- The panel includes a new standing order protocol which our nursing colleagues will follow to assess any contraindications to the use of mupirocin. (Click here to access the Nasal Decolonization Protocol [Requires VUNetID])
- Mupirocin administration will be charted on the MAR like other medications, making it easier to track number of dose given and due (as patient’s require a 10 dose course [twice a day for 5 days])
- A short education module (<5 mins) will be assigned to personnel
An updated FAQ sheet is also available HERE
-
Ebola Virus Disease

Contact 615-875-4000 and activate the EBOLA RESPONSE TEAM if you encounter a patient in which Ebola infection is suspected.
CLICK HERE FOR MORE INFORMATION.
-
MPOX UPDATE
MPOX SPECIMEN COLLECTION FAQs (UPDATED - 8/31/2022)
TN DOH SPECIMEN COLLECTION INSTRUCTIONS (CLICK FOR INSTRUCTIONS)
Clusters of Mpox cases have been reported in several countries over the past year, including in the United States. Previous cases outside of Africa have been associated with travel from Nigeria, though recent cases do not have direct travel-associated exposure risks. There is evidence to suggest that some cases may be epidemiologically linked (i.e., the patients were identified at sexual health clinics).
Clinicians are asked to keep a high suspicion for potential cases and to follow these important steps to prevent mpox virus transmission at VUMC:
- Consider mpox in patients presenting with a new vesicular or pustular rash. Suspicion should be heightened if the rash occurs in people who within 21 days of illness onset:
- Reports having contact with a person or persons with a similar appearing rash or with a person who has received a diagnosis of confirmed or probable mpox OR
- Had close or intimate in-person contact with persons in a social network experiencing mpox infections. This includes MSM who meet partners through an online website, digital application (“app”), or social event (e.g., a bar or party) OR
- Traveled, within 21 days of illness onset outside the United States to a country with confirmed cases of mpox or where mpox virus is endemic OR
- Had contact with a dead or live wild animal or exotic pet that is an African endemic species, or used a product derived from such animals (e.g., game meat, creams, lotions, powders, etc.)
- Presenting symptoms typically include fever (but this has been less common in the current outbreak), chills, the distinctive rash, or new lymphadenopathy; however, onset of perianal or genital lesions in the absence of subjective fever has been reported.
- The rash associated with mpox involves vesicles or pustules that are deep-seated, firm or hard, and well-circumscribed; the lesions may umbilicate or become confluent and progress over time to scabs.
- The rash associated with mpox can be confused with other diseases that are more commonly encountered in clinical practice (e.g., secondary syphilis, herpes, chancroid, and varicella zoster).
- Transmission of the virus can occur through contact with body fluids, mpox sores, items that have been contaminated with fluids or sores (clothing, bedding, etc.), or through respiratory droplets following prolonged face-to-face contact.
- For any suspected mpox case:
- Place patient on Contact and Airborne Precautions. A negative pressure room is only necessary for patients who will be undergoing aerosol-generating procedures. All persons who enter the room must wear gloves, gown, eye protection and an N95 respiratory or PAPR. Limit room entry to only essential personnel.
- VUH/MCJCHV/VBH/VMG Clinics: Notify the VUMC Department of Infection Prevention at 615-835-1205 (24/7) immediately. VWCH/VTHH/VBCH: Notify your nursing supervisor immediately who should then contact the local Infection Prevention on-call number (24/7).
Please be advised that these issues are evolving and advice may change as new information arises. For questions or additional information, please contact the VUMC Department of Infection Prevention. More mpox information is available at https://www.cdc.gov/poxvirus/monkeypox/index.html.
INSTRUCTIONS FOR PATIENTS:
- Guidance if Exposed to Someone with Mpox
- Consider mpox in patients presenting with a new vesicular or pustular rash. Suspicion should be heightened if the rash occurs in people who within 21 days of illness onset:
-
Middle-East Respiratory Syndrome Coronavirus (MERS-COV)
More information can be found below:
Tennessee Department of Health Alert (May 1, 2014)
Centers for Disease Control and Prevention
Specimen Testing:
MERS testing at TN DoH and/or CDC should begin with consultation by a state epidemiologist, prior to specimen collection. Please contact a member of the microbiology services if MERS is under consideration and prior to specimen to collection. One of the microbiologists will coordinatewith the clinical team and specimen-receiving lab to support safe and efficient specimen handling for eventual routing to the state laboratory. If/when the state epidemiologist approves testing, the specimen(s) should be collected per instructions and submitted to our central receiving lab with the following documentation:
- VUMC test requisition, state specimen submission form
- CDC specimen submission form
Please VERY CLEARLY indicate the suspicion for MERS on the test requisition to help avoid unintentional testing or opening of the specimen in the VUMC laboratory. If the DDx expands to include MERS after respiratory specimens have been collected and submitted to the laboratory for other testing, please immediately contact the microbiology service so that we can assist in the safe management of those materials pending a determination of whether the patient meets PUI criteria.
-
Prevent The Spread of Influenza

VUMC policy requires annual influenza vaccination or exemption. Exemptions may be for religious or personal/philosophical beliefs or for medical contraindications. Those who wish to be exempted from receiving the flu vaccine must complete an exemption form, available on the OHC website beginning in September. Exempted personnel must wear a mask during influenza season as noted in the VUMC Immunization policy. More details on masking may be found here.
Do you never get an annual influenza vaccination?
Do you come to work with a "cold?"
If you answered "yes," you could be spreading infleunza to
your patients and colleagues (even when you don't feel sick!)
It's influenza season again -- find out how you can protect yourself,
your colleagues, and your patients from influenza.
-
The diagnosis of Clostridiolides difficile infection (CDI) requires the detection of bacterial toxin and/or antigens in the stool. Due to the need to better assess whether a patient has active CDI vs. stool colonization of a toxin-capable strain, the VUMC lab is migrating to a multistage reflexive test for CD. The previous CD test was a molecular PCR test that only could detect whether the patient had a strain of CD that could produce toxin (which causes clinical disease) NOT whether the toxin was being actively produced. Hence, there was a risk that a patient who was merely colonized with toxin-capable CD strains but had diarrhea due to other causes (e.g. tube feeds, laxatives) would be misdiagnosed as having CD infection and treated as such. The new test is a multistage test that first looks for the toxin gene by PCR (just like the old test) but then, if the gene is present ("PCR Positive"), the second test looks for active production of the toxin ("PCR and Toxin Positive").
Information about new C. difficile test - results format
Information about new C. difficile test - isolation
-
Urine Culture Testing Algorithm
In order to reduce the risk of false positive/contaminated urine cultures and to help better guide clinicians in the interpretation of positive urine cultures, VUMC has implemented a standardized urine culture testing process (known as U/A with reflexive culture) as part of a multi-part program to reduce variability around urine cultures. This program also includes guidance on the indications for urine culture ordering that will be presented on order entry, standardized specimen collection protocols, and tracking of urine culture contamination rates.
For the U/A with reflexive culture process, a U/A will be sent with every culture specimen order. Clinicians will be asked on order entry if the patient has a recognized condition that could either impact the U/A interpretation or where national guidelines recommend treating positive cultures even in the absence of positive U/A results. These are 1) pregnant patients, 2) patients undergoing urologic surgery or with complex GU history at high risk for UTI, 3) neutropenic patients, and 4) children under 25 months of age. In the absence of any of these conditions, if the U/A is negative (defined as negative nitrites, less than small leukocyte esterase, and <5 WBC/hpf), then the urine culture will not be processed. If the U/A is positive, the urine culture will be processed without requiring any additional action from the ordering clinicians. If the clinician notes the presence of any of the 4 conditions listed above, they will have the option of ordering either a U/A with urine culture or urine culture alone. For clinicians in outpatient areas, an additional exclusion to the reflex process is in the even that a point of care test indicates presence of a UTI (e.g. urine dipstick).
The test can be ordered by selecting U/A or urine culture and selecting the U/A with reflexive culture option. You do not need to order the U/A as a separate test, as it is part of the reflexive testing. For more information about the urine culture standardization, please click on the links below:
Urine Culture Standardization Summary (6/14/2016)
-
VUMC Named First APIC Program of Distiction in Infection Prevention!

Vanderbilt University Medical Center (VUMC) is the first hospital system in the nation to receive the Association for Professionals in Infection Control and Epidemiology (APIC) Program of Distinction designation, an acknowledgement of excellence for infection prevention and control programs that meet stringent standards established by the association.
The designation is the culmination of an intensive review process that began last summer when an APIC survey team visited VUMC to evaluate infection prevention practices at Vanderbilt University Adult Hospital and Monroe Carell Jr. Children’s Hospital at Vanderbilt as well as numerous off-site locations.
APIC is the leading professional association for infection preventionists (IPs) in the United States, with more than 15,000 members. APIC’s Program of Distinction designation measures excellence in infection prevention policies and procedures and ongoing quality improvement efforts, as well as compliance with federal regulations.
For more information: CLICK HERE
APIC Program of Distinction Home Page
Vanderbilt Medicine article on the VUMC IP Program and POD Designation
-
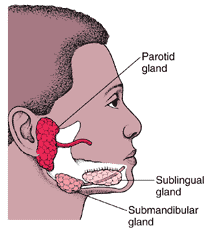
Clinical Presentation and Illness Course
What is mumps?
Mumps is a viral illness best known for the puffy cheeks and swollen jaw that it causes. This is a result of swollen salivary glands.
How does a case of mumps present?
The most common symptoms include fever, headache, muscle aches, tiredness, loss of appetite, and swollen and tender salivary glands under the the ears on one or both sides (parotitis). The gland swelling may occur several days after the onset of the other symptoms.
Some people who get mumps have very mild or no symptoms, and often they do not know they have the disease.
When do symptoms appear?
Symptoms typically appear 16-18 days after infection, but this period can range from 12-25 days after infection.
How serious is mumps?
Most people recover completely in a few weeks.
Rarely, complications such as orchitis (inflammation of the testes), masitis (inflammation of the breast), pancreatitis, encephalitis, and menigitis can occur. Death from mumps is exceedingly rare.
Transmission
How does the mumps virus spread?
The mumps virus infects cells in the upper respiratory tract and spreads throughout direct contact with respiratory secretions or saliva or through fomites (e.g. hands).
The risk of spreading the virus increase the longer and the closer the contact a person has with someone who has mumps.
When a person is ill with mumps, he or she should avoid contract with others from the time of diagnosis until at least 5 days after the onset of parotitis by staying home from work or school and staying in a separate room if possible.
Patients who are suspected of having mumps infection should be placed into Droplet Precautions (wear a surgical mask to enter patient room).
Diagnosis
What specimens should be collected from patients who meet the mumps clinical case definition?
The TN Dept of Health (DOH) recommends that a buccal (inner cheek) swab collected using a Dacron swab placed into viral transport media be collected from all patients with clinical features compatible with mumps. These should be sent for mumps PCR and culture testing. This test must be approved by the DOH - this approval can be facilitated by the Department of Infection Prevention.
Serology testing (for IgM) can become positive later in the course of illness but should not be used alone to determine the presence of acute mumps infection. In addition, commercial serology testing on someone who is previously vaccinated is very difficult to interpret. True mumps cases with past mumps immunization can have negative IgMs; there are issues with cross-reacting antibodies in some cases of infection with other viruses; and, in situations where a person has a high IgG they can have false positive IgM due to the incomplete clearance of IgG in the commercial indirect capture assay. Therefore, the TN DOH does not recommend using mumps serology to diagnose an acute case of mumps in a vaccinated individual. See https://www.cdc.gov/mumps/lab/qa-lab-test-infect.html for more information.
Remember that other viruses (including influenza A which is currently circulating in Middle Tennessee) can cause parotitis. Send respiratory viral panel testing to rule out such infections in suspected cases.
How do I collect a buccal swab?
Massage parotid gland for 30 seconds (or submandibular gland if that is the symptomatic gland)
Swab area around Stenson's duct (or Wharton's duct if submandibular gland)
Use the flocked swab that is packaged with the Viral Transport Medium (VTM)
Break off the swab and leave it in the VTM. The swab should remain in contract with the VTM for at least an hour.
Be sure to mark the VTM tube with "BUCCAL" to avoid confusing it with the nasopharyngeal swab.
Details here: https://www.cdc.gov/mumps/lab/detection-mumps.html
Vaccination
Is there a vaccine against mups?
Yes. Mumps can be prevented with MMR vaccine. This protects against three diseases: measles, mumps, and rubella. CDC recommends children get two doses of MMR vaccine, starting with the first dose at 12 through 15 months of age, and the second dose at 4 through 6 years of age. Teens and adults should be up to date on their MMR vaccination.
MMR vaccine is very safe and effective. The mumps component of the MMR vaccine is about 88% (range: 66-95%) effective when a person gets two doses.
Compliance with MMR vaccination/immunity is very high among VUMC faculty, staff and students, which will help stem transmission.
When would CDC recommend giving individuals a 3rd MMR does?
Although evidence of its effectiveness is needed, a third of dose of MMR vaccine may be considered as a control measure during mumps outbreaks occurring in settings in which persons are in close contact with one another, when transmission is sustained for over 2 weeks despite high 2-dose MMR coverage, and when traditional control measures to fail to slow transmission. The Department of Health is not currently recommending a 3rd MMR dose for any individuals involved with this current cluster.
Occupational Health Clinic Recommendations
- Recognize signs and symptoms of mumps and follow the infection control precautions outlined above.
- Know your immune status: Healthcare workers are considered immune if they have:
- documentation of 2 doses of mumps/MMR vaccine after age 12 months
- Blood test that show immunity
- Birthdate before 1957
- Occupational health will identify and contact exposed individuals, determine immunity status, and provide any additional recommendations.
- Check your own vaccination/immunity status in the Health and Wellness Information Portal at https://myhealthandwellness.vanderbilt.edu. Click "Occupational Health Status" to see what services are needed for compliance, and "Occupational Health Record" to view your record.
- Anyone without evidence of immunity in their record can come to the Occupational Health Clinic (hours: 7:30a - 5:30p M-F) to receive the vaccine.
- For any questions, please contact the Occupational Health Clinic at 615-936-0955
Recommendations for all VUMC Clinicians
- Consider mumps as a diagnosis in anyone with unilateral or bilateral partoid or other salivary gland swelling.
- Isolate suspected mumps causes using Droplet Precautions (wear a surgical mask to enter patient room) and place surgical mask on the patient when he or she must travel outside of the room.
- Obtain specimens for testing (oral/buccal swab for mumps PCR if Approved by DOH, mumps serology IgM and IgG if unvaccinated, other viral testing such as RVP).
- All healthcare workers need to be up to date on MMR vaccine/or immunity status. Check your vaccine/immunity status in the Health and Wellness Information Portal as outlined above.
Please contact Infection Prevention with any questions 615-835-1205.
-
Zika Virus Guidance & Resources (Updated 8/1/2016)
Zika General Information
TN Dept of Health Update (5/10/16)
TN Dept of Health Update (6/2/16)
TN Dept of Health Update (8/1/16)
Zika & Pregnancy: CDC Guidelines
Testing Form (Specimens sent to CDC via TN State Lab): Please note the lab client services number, 5-LABS (615-875-3227) on the test requisition as contacts for questions from the receiving lab. TDH will perform PCR and serology (IgM, IgG) for CHIK and forward specimens to CDC for Zika testing, which may involve a combination of serology, PCR, and culture-based demonstration of Zika-specific neutralizing antibodies. Note: per CDC, Because Zika virus testing is not listed in the drop-down menu for the Test Order Name field of form 50.34 (located on 1st page, top left), you will need to select ARBOVIRUS SEROLOGY and then type Zika testing in the Brief Clinical Summary field located at the top of the second page of the form. According to the CDC web site, results are usually available 4-14 days after specimen receipt, longer during summer months. A positive initial serologic screen for Zika will trigger confirmatory testing, which may delay final results. A report hardcopy will be available ~2 weeks after test completion and communicated directly to TDH Lab.




