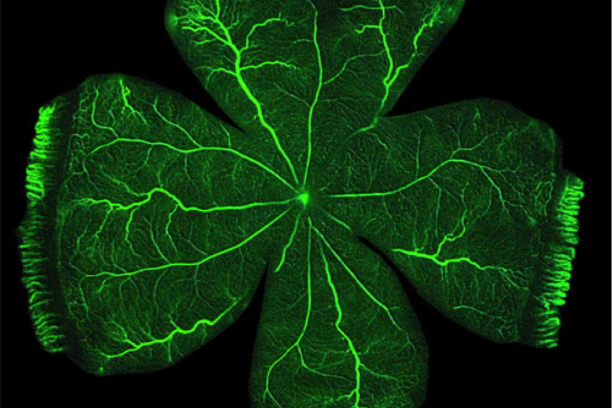
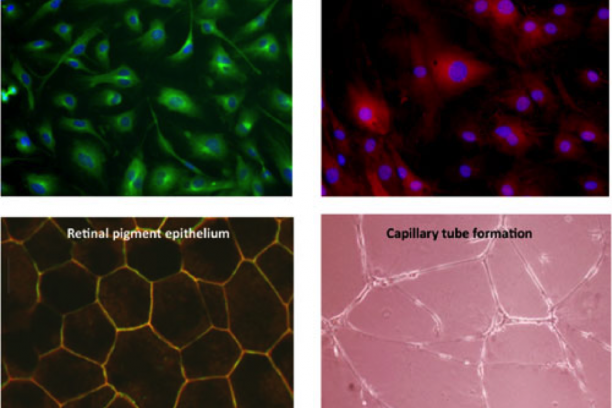
Vascular Biology of the Retina
The large majority of irreversible blindness in the US is caused by pathology of the blood vessels of the eye. Penn's long-standing interest is in the molecular basis of retinal vascular disease. The over-arching goal of his research is to characterize the processes involved in retinal vascular inflammation and angiogenesis, and to begin to develop preventive strategies based on understanding gained from in vitro and in vivo studies. The Penn lab has the capability to isolate and culture a variety of primary cells from retinal tissue of several species, including retinal vascular endothelial cells, choroidal endothelial cells, retinal Müller glia, retinal pericytes, retinal microglia and retinal pigment epithelial cells – all of which are involved in vascular diseases of the retina. In addition, the lab uses a battery of in vivo models of vascular diseases of the eye including rodent models of retinopathy of prematurity, neovascular age-related degeneration and diabetic retinopathy. Using these in vitro and in vivo tools, Penn's research program focuses on pro-inflammatory and pro-angiogenic molecular signaling in the retina. In his research, Penn places an emphasis on both drug target identification and on development of novel pharmaco-therapeutics and methods of drug delivery to the eye.
Penn has been continuously funded by the National Eye Institute of NIH for 33 years. He currently serves on the advisory boards of three prominent foundations supporting eye research, and he is President of the International Society for Eye Research.
Through an enterprise called VO-CRO (Vanderbilt Ophthalmic Contract Research Organization), Penn’s lab currently holds 18 contracts with pharmaceutical companies to develop novel drugs for eye diseases. More information about VO-CRO can be found here: VO-CRO website
An overview of the Penn lab's research interests and current projects can be found here: Penn lab overview
-
NFAT and retinal vascular homeostasis: The Penn lab currently has multiple projects directed at understanding the role of the transcription factor, NFAT, in diabetic retinopathy pathogenesis. First, we are investigating NFAT’s regulation of extracellular matrix expression in the development of basement membrane thickening – a hallmark of diabetic retinopathy. Second, we are characterizing the role of NFAT in the response of photoreceptors to diabetes-relevant stimuli. Finally, we are determining NFAT isoform specificity in early pathogenic events related to retinal cytokine induction under diabetic conditions.
Epoxygenated lipids and their products in retinal vascular inflammation: Epoxide lipids generated by Cytochrome P450 epoxygenases are anti-inflammatory, but their levels are limited by the soluble epoxide hydrolase enzyme. We are testing the hypothesis that epoxide levels are decreased in the diabetic retina due to altered expression of the enzymes that regulate them, and therefore elevating their levels would be beneficial for the early treatment of diabetic retinopathy. Similarly, the lipid epoxide-derived endocannabinoids that are selective for cannabinoid receptor 2 (CB2) binding are also potently anti-inflammatory. We are characterizing their roles in diabetic retinopathy and the efficacy associated with elevating their endogenous levels.
Novel biomarker imaging and drug delivery methods: The properties of the eye allow for unique opportunities to image biomarkers in vivo. We are designing novel RNA-based molecular beacons and contrast agents to better understand early disease processes, and as a first step in developing targeted strategies for delivery of novel therapeutic agents.
-
Faculty
John S. Penn, Ph.D.

Dolly A, Padovani-Claudio, M.D., Ph.D.

Dolly Ann Padovani-Claudio, M.D., Ph.D, is a physician-scientist in the pediatric ophthalmology division where she cares for children with ocular conditions. Her research aims to better understand the role of select chemokines, such as IL-8, in mediating inflammation and new vessel growth in the retina. IL-8 is elevated in the vitreous of patients with diabetic retinopathy, so the goal of Dr. Padovani-Claudio's project is to determine whether inhibitors of the IL-8 receptor, CXCR1/2, will be effective in pre-clinical models of diabetic retinopathy with the goal of quickly translating these therapeutics to the clinic.
dolly.a.padovani-claudio@vumc.org
Imam Uddin, Ph.D.

Imam Uddin, Ph.D, is a research instructor developing nanotechnologies and novel optical imaging methods to detect molecular changes in real-time, as well as silencing specific gene targets with nanoparticles. He is synthesizing oligonucleotide-conjugated nano-gold colloids for imaging and silencing levels of specific mRNA targets in living cells and tissues. He is currently investigating this new technology in animal models of common eye diseases. In addition, Uddin has developed HYPOX-4, a novel fluorescence-imaging probe capable of detecting retinal hypoxia in living animals.
Irina De la Huerta, M.D., Ph.D.

Dr. Irina De la Huerta, M.D., Ph.D, is an assistant professor and a physician scientist caring for adults and children with retinal diseases. Her research investigates the hypothesis that photoreceptors, the light-sensing cells in the retina, accelerate the development of retinal vascular diseases such as diabetic retinopathy. She is combining advances in cell culture techniques, genetic and metabolic animal models, and high-throughput biology, with the overall goal of developing novel therapeutic interventions that target the early stages of diabetic retinopathy.
Postdoctoral Fellows
Cayla Ontko, PhD

Cayla Ontko is interested in studying the effects of epoxygenated fatty acids and endocannabinoids on retinal vascular inflammation in DR. Recent evidence has shown a positive correlation between retinal inflammation and DR progression/vision loss. Cayla studies the inflammatory pathways of DR to gain insight into better therapeutic intervention and therefore vision preservation in adults with diabetes. Currently she is focusing her studies on epoxygenated fatty acids and endocannabinoids, both of which may have anti-inflammatory potential.
Graduate Students
Amy Stark

Staff
Gary McCollum, PhD; Senior Scientist
Taylor Smith, MAcc; VO-CRO Financial Analyst
Rong Yang, MD; RA III
Rachel Jerrell; Lab Manager
Research Assistants: Anna Gilmartin, Greta Galli, Lindsey Van Metre, Maximilian Garcia, Blake Dieckmann
VOCRO Project Managers: Jazzmine Faust, Isabelle Newkirk, Mariana Gabi-Miles, PhD
Administrative Assistant: Kyana Arellano
Tim Lee, PhD- Staff Scientist
Undergraduate Students
Oremeyi Daniyan
Sophia Salgueiro
Sebastian Bujoi
Nitya Kodali
-
Giblin, M.J., Smith, T.E., Winkler, G.A., Pendergrass, H., Kim, M.J., Capozzi, M.E., Yang, R., McCollum, G.W. and Penn, J.S. (2021) Nuclear Factor of Activated T-cells (NFAT) regulation of IL-1β-induced retinal vascular inflammation. Biochim Biophys Acta Mol Basis Dis. 2021 Dec 1;1867(12):166238. PMC8565496.
Giblin, M.J., Ontko, C.D. and Penn, J.S. (2022) Cytokine-induced alterations in extracellular matrix composition contribute to DR-relevant endothelial cell behaviors. Nature Sci Rep. 12, 12955. PMC35902594.
Paguaga, M., Penn, J.S., Uddin, M.I. (2023) A novel optical imaging probe for targeted visualization of NLRP3 inflammasomes in a mouse model of age-related macular degeneration. Front. Med. Volume 9:1047791. PMC9871584.
Dieckmann, B.W., Paguaga, M.E., McCollum, G.W., Penn, J.S., Uddin, I. (2023) Role of NLRP3 inflammasomes in monocyte and microglial recruitments in choroidal neovascularization. Immunohorizons. 8(5):363-370. PMC11150128.
Atalor, R., Dieckmann, B., Penn, J.S. and Uddin, M.I. (2023) A method to regulate monocyte function by silencing HIF-1α mRNA in a model of retinal neovascularization. ACS Appl. Nano Mater. 6, 24, 22939–22946. DOI: 10.1021/acsanm.3c04300.
Padovani-Claudio, D.A., Ramos, C., Capozzi, M.E. and Penn, J.S. (2023) Elucidating glial responses to products of diabetes-associated systemic dyshomeostasis. Prog Ret Eye Res. 94:101151; PMC10683564.
Stark, A.K. and Penn, J.S. (2024) Prostanoid signaling in retinal vascular disease. Prostaglandins Other Lipid Mediat. 174:106864. PMID: 38955261. https://doi.org/10.1016/j.prostaglandins.2024.106864.
Jamal, S.Z., Diekmann, B.W., McCollum, G.W., Penn, J.S., Jayagopal, A. and Uddin, M.I. (2024) Imaging hypoxia to predict primary neuronal cell damage in branch retinal artery occlusion. Microcirc. 31:7, e12892. PMID: 39213162.
Padovani-Claudio, D.A., Morales, M.S., Ontko, C.D., Smith, T.E., Namburu, N.S., Palmer, S.A., Jhala, M.G., Ramos, C.J., Capozzi, M.E., McCollum, G.W. and Penn, J.S. (2024) Induction, amplification, and propagation of diabetic retinopathy-associated inflammatory cytokines between human retinal microvascular endothelial and Müller cells and in the mouse retina. Cell. Signal. https://doi.org/10.1016/j.cellsig.2024.111454.
Stark, A.K. and Penn, J.S. (2024) Prostanoid signaling in retinal cells elicits inflammatory responses relevant to early-stage diabetic retinopathy. J. Neuroinflam. (In press).
Ontko, C.D., Smith, T.E., Stark, A.K., Olson, J.C., Newkirk, I.M., Jackson, A.L., McCollum, G.W. and Penn, J.S. (2024) Cannabinoid Receptor 2 Agonism Demonstrates Therapeutic Potential in Experimental Models of Relevance to Diabetic Retinopathy. Diabetes (In review).
-
- R01 EY007533 - Molecular Mechanisms of Retinal Vascular Disease
- R01 EY023397 - In vivo Molecular Imaging of the Retina
- R01 EY023639 - The Calcineurin/NFAT Signaling Axis in Diabetic Retinopathy Pathogenesis
- R01 EY029693 - In Vivo Molecular Imaging of Vascular Disease of the Retina
- K08 EY029006 - Retinal Vascular Inflammation and Angiogenesis in Diabetic Retinopathy
- Reeves Foundation
- Research to Prevent Blindness
- Jean Love Foundation
- Phyllis G. and William B. Snyder Foundation
- 18 current research contracts with drug companies