See also the Managing Particularly Hazardous Substances and Peroxide Forming Chemicals factsheets.
print version of this document
Introduction
Prudent chemical retention and storage practices are vital to maintain a safe laboratory working environment and to minimize the financial costs and environmental impact associated with the handling and disposal of unwanted chemicals. The American Chemical Society endorses the "Less Is Better" (1993) approach which emphasizes the safety and financial reasons for buying chemicals in small packages on an as needed basis: reduced risk of breakage, reduced risk of exposure following accidents, reduced storage costs, reduced wasted from decomposition during prolonged storage in partially empty bottles, and reduced disposal cost for unused materials. For chemicals likely to be used in the near future, a well-managed storage plan is necessary in order to reduce the risk of incompatible chemical reactions and unwanted exposures to particularly hazardous substances. This fact sheet focuses on proper guidelines for chemical retention and storage.
Chemical Retention
Temperature, humidity, light, exposure to air and other substances are several factors that affect chemical purity and can contribute to chemical decomposition. Decomposition can lead to the formation of hazardous reactive chemical by-products. It may also affect the quality of research when decomposed chemicals turn into unknown or unintended compounds. The following are general recommendations for chemical retention:
- The date the material was received and the date the container was first opened should be recorded. This is especially important to track those chemicals that degrade rapidly and/or form explosive peroxides.
- Organic peroxides are a class of compounds that have unusual stability problems that make them among the most hazardous substances found in the laboratory. As a class, organic peroxides are considered to be powerful explosives and are sensitive to heat, friction, impact, and light, a well as to strong oxidizing and reducing agents. Please refer to the OCRS fact sheet Peroxide Forming Chemicals for more detailed information for handling and storage of peroxide forming chemicals.
- Properly dispose of chemicals and gases and their empty containers.
- Properly dispose of any chemicals or gas cylinders past an expiration date listed on the label or that have been stored beyond the shelf life recommendations given on the supplier MSDS or technical datasheet.
- Properly dispose of any unlabeled chemical containers or gas cylinders. Unlabeled chemicals are not only a danger to lab staff but also to housekeeping and emergency personnel.
- Submit waste collection requests on the OCRS website.
- Keep a current inventory of all chemical compounds and compressed gas cylinders in the laboratory. Vanderbilt University has a tool for assistance with maintaining chemical inventories.
Chemical Storage
FLAMMABLE LIQUID STORAGE
Flammable liquids should be stored in flammable liquid storage cabinets or inside a designated flammable liquid storage area. The maximum volume of flammable liquids allowed in laboratories outside flammable liquid storage cabinets are as follows (NFPA 45):
- 10 gallons (38 liters) of Class 1 flammable liquids per 100 sq. ft. area. (Flash point < 100˚F)
- 20 gallons (78 liters) of Class I, II, and IIIA flammable liquids. (Flash point > 100˚F for Class II and > 140˚F for Class IIIA).
- An additional 10 gallons of Class 1 flammable liquids can be stored in a flammable liquid storage cabinet. Combinations of Class 1, Class II, and Class IIIA flammable liquids may not exceed 40 gallons in a flammable liquid storage cabinet.
- Flammable-liquids storage cabinets are not intended for the storage of highly toxic materials, acids, bases, compressed gases or pyrolytic chemicals.
- The maximum quantity of flammable and combustible liquids allowed in a properly designed and protected flammable liquid storage room is 5 gallons (19 liters) per square foot of floor area (NFPA 30).
- Purchase the smallest volume container needed for research. This is especially important with glass containers storing flammable liquids since these are highly susceptible to breakage.
- Large bottles should be stored low to the ground in order to prevent large spills from dropping.
STORAGE OF PARTICULARLY HAZARDOUS SUBSTANCES (PHS)
- Particularly Hazardous Substances (PHS) should be segregated from other less hazardous chemicals in the laboratory. PHS includes regulated substances, known carcinogens, reproductive hazards, sensitizers, highly acute toxins, or highly corrosive chemicals.
- For more information, refer to the OCRS Fact Sheet on “Managing Particularly Hazardous Substances (PHS) In Your Laboratory” available on the OCRS website.
STORAGE OF CHEMICAL HAZARDOUS WASTE
- Storage of Hazardous Waste in the Laboratory: Each lab should have a designated location in which to store hazardous materials to be discarded (do not keep radioactive waste and hazardous chemical waste in the same place). This location should be out of the way of normal lab activities, but easily accessible and recognizable by OCRS staff.
- Refer to the Laboratory Guide for Managing Chemical Waste available on the OCRS website.
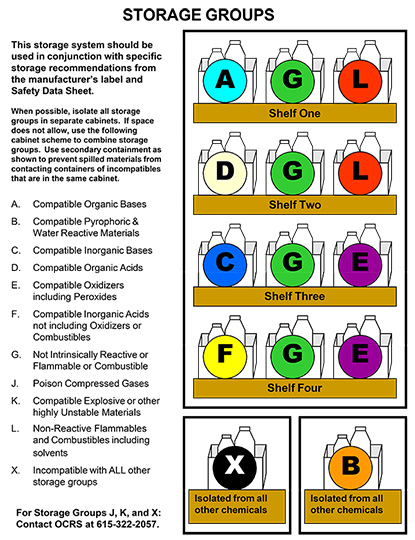
CHEMICAL STORAGE GROUPS
Chemicals are best segregated by hazard class to avoid incompatibilities. DO NOT STORE CHEMICALS ALPHABETICALLY, except within a hazard class. Plastic bins can be used to provide secondary containment and segregation on shelves. Recommended general hazard classes for storage are listed below. Chemtracker can assist with the designation of storage groups for particular chemicals:
A - Compatible Organic Bases
Examples: hydroxylamine, tetramethylethylamine diamine, triethylamine, phenylhydrazine
B - Compatible Pyrophoric & Water Reactive Materials
React with water to yield flammable or toxic gases. Examples include sodium, potassium, metal hydrides and hydrolysable halides (titanium tetrachloride, phosgene etc.) Keep away from water sources. Do not store above or below sinks. Use dry chemical extinguisher for fire.
C - Compatible Inorganic Bases
Materials with a pH > 9. Examples include ammonium hydroxide, calcium hydroxide, and sodium hydroxide. Separate from acids. Store solutions of inorganic hydroxides in polyethylene containers.
D - Compatible Organic Acids
Examples: propionic acid, trichloroacetic acid, acetic anhydride, acetyl bromide. Separate from inorganic acids.
E - Compatible Oxidizers including Peroxides
React with water, fire, flammables and combustibles. Examples include inorganic nitrates (nitric acid), permanganates, inorganic peroxides, persulfates, and perchlorates (perchloric acid). Keep separate from flammables and other organic materials. Keep separate from reducing agents (i.e., zinc, alkaline metals, and formic acid). Do not store directly on wooden surfaces.
F - Compatible Inorganic Acids not including Oxidizers or Combustibles
Materials with pH < 5. Examples include hydrochloric and hydrofluoric acid. Separate from active metals including sodium and potassium and from organic acids.
G - Not intrinsically Reactive or Flammable or Combustible
Example: NaCl, buffer solutions
J* - Poison Compressed Gases
Example: Hydrogen sulfide, chlorine
K* - Explosive or other highly unstable materials
Example: Picric Acid, nitrocellulose
L - Non-Reactive Flammables and Combustibles, including solvents
Flammable/Combustibles vapors ignite easily at room temperature. Examples include alcohols, esters, ketones, ethers and pyrophorics. Store flammable liquids in approved safety cans or cabinets. Keep away from heat, sun, flame, and spark sources. Separate from oxidizers. See Flammable Liquid Storage section.
X* - Incompatible with all other storage groups
*Storage Groups J, K, and X are particularly hazardous and are incompatible with all other storage groups, or require special storage considerations. For assistance with these storage groups, please contact OCRS (322-2057).
Download the Storage Groups as a full size PDF file suitable for printing.