Lars Plate, PhD
More Information
Chemical Biology, Biochemistry, Cell Biology, Proteomics, Microbiology, Enzymology, Drug discovery, VICB
Lars grew up in northern Germany and first moved to the USA to earn his BS in Chemistry at the Massachusetts Institute of Technology. He conducted research in the group of JoAnne Stubbe where he investigated the subunit interactions of the enzyme ribonucleotide reductase. For his doctoral work, Lars studied bacterial nitric oxide gas sensing proteins and signal transduction pathways with Michael Marletta at the University of California, Berkeley. He completed his PhD in Molecular and Cell Biology with emphasis in Chemical Biology in 2013. He then joined the groups of Jeff Kelly and Luke Wiseman at the Scripps Research Institute in La Jolla, CA for his postdoctoral training, where he used chemical biology and proteomics approaches to investigate endoplasmic reticulum (ER) proteostasis pathways and protein quality control mechanisms. He defined how these processes can be targeted therapeutically to treat diverse protein misfolding and aggregation diseases using newly identified small molecule ER proteostasis regulators. Lars joined the faculty at Vanderbilt in 2017.
Research Information
The focus of the group is to define the dynamics and the coordination of protein interaction networks in diverse cellular processes. Towards this goal, we develop new mass spectrometry-based proteomics and chemical biology tools. Dynamic protein-protein interactions govern many molecular, cellular, and organismal processes and altered interactions are intricately linked to disease states. For instance, in cancer disparate protein-protein interactions occur in signal transduction pathways, protein folding diseases arise from aberrant engagement of protein folding pathways, and pathogenic infections take advantage of host-pathogen protein interactions that co-opt cellular pathways. Our goal is to understand how protein interactions in the pertinent biological processes have to be properly timed and coordinated. Defining the pathological consequences of mistimed and uncoordinated protein interactions on disease states will then guide the identification of new therapeutic strategies.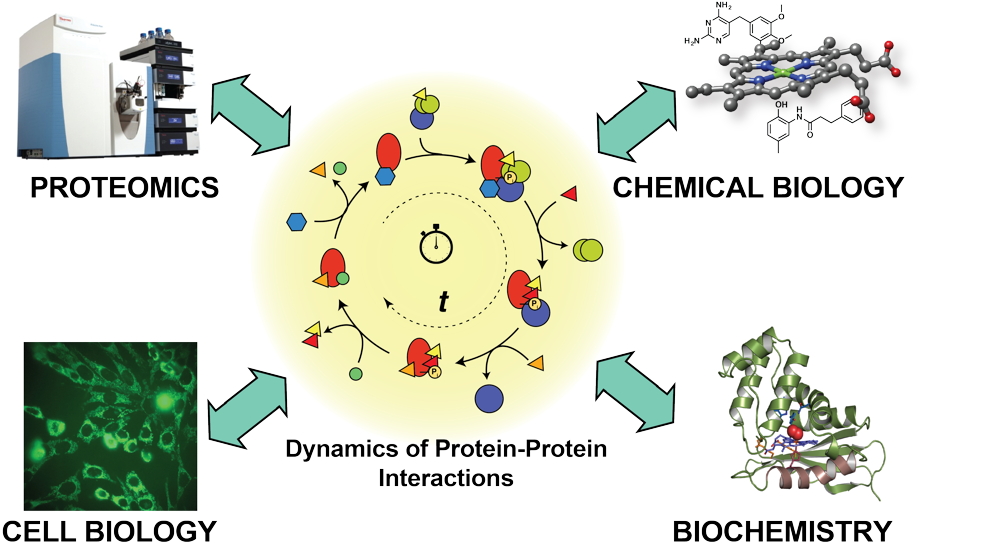
The research in our group leverages multidisciplinary approaches at the interface of Chemistry and Biology, ranging from protein biochemistry and enzymology, to microbiology, and cell biology as far as proteomics and drug discovery.
