At VO-CRO, our focus is on providing the best and most translatable research possible. To that effect, the use of primary cells is very important to us. We have years of experience isolating and culturing multiple cell types from different species including mouse, rat, and human. As manipulation of targets of interest is also critical to research, we have developed methods to efficiently transfect each of these cell types. We use a wide variety of cells in order to determine the relative contribution of each cell type in disease onset, progression and regression.
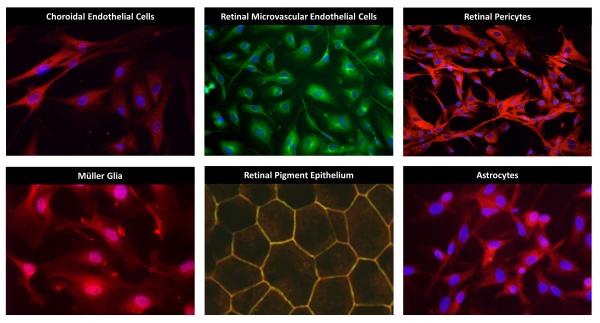
Choroidal Endothelial Cells
The choroid is a fenestrated vascular bed that provides nutrients to the photoreceptors. These cells are particularly important in age-related macular degeneration. These cells can be treated with disease-relevant stimuli to induce expression of cytokines and growth factors allowing for the careful examination of pro-inflammatory and pro-angiogenic signaling pathways.
Retinal Microvascular Endothelial Cells
RMEC comprise the microvascular network of the inner retina. Their main role is to provide nutrients to retinal cells while maintaining the blood-retina barrier. Like choroidal endothelial cells, retinal microvascular endothelial cells can be stimulated to express gene products that are important in disease pathogenesis. Using in vitro methods, the molecular pathways involved in disease pathogenesis can be examined under tightly controlled experimental conditions.
Retinal Pericytes
Pericytes are the support cells of the vasculature. Normal pericyte function is critical to the survival of microvascular endothelial cells under pathologic conditions and to appropriate regulation of vascular permeability. Human pericytes can be cultured to examine regulation of apoptotic pathways, cytokine production and other cell behaviors leading to disease onset and progression.
Müller Glia
Müller cells are specialized glial cells that span the thickness of the retina. They are responsible for retinal homeostasis and are the main producers of cytokines and growth factors in disease. For example, Müller cells produce many-fold more VEGF, TNF-alpha, interleukins and other growth and inflammatory factors than all other retinal cell types combined. We culture these cells to explore the signal pathways and intermediates that regulate production of these and other factors under disease-relevant conditions.
Retinal Pigmented Epithelium
RPE is a layer of pigmented epithelial cells responsible for the permeability barrier between the retina and the choroid. RPE are also important sources of growth factors and cytokines that promote disease progression. We culture and study RPE in these two contexts, using TEER, ELISA, qRT-PCR and other methods.
Astrocytes
Retinal astrocytes maintain homeostasis. Because astrocytes can express and secrete growth factors and cytokines, they are among the battery of cells in which we examine these functions under disease-relevant conditions. Our overall purpose is to determine the relative contributions of the various retinal cell types in disease onset, progression and regression.
