As the population ages, late-onset Alzheimer's disease (AD) is becoming an increasingly important public health issue. Clinical trials targeted at reducing AD progression have demonstrated that patients continue to decline despite therapeutic intervention. Thus, there is a pressing need for new treatments aimed at novel therapeutic targets. A shift in focus from risk to resilience has tremendous potential to have a major public health impact by highlighting mechanisms that naturally counteract the damaging effects of AD neuropathology. Interestingly, at autopsy, approximately 30% of cognitively normal individuals have the pathological features of AD. Research from our group has begun to uncover genetic factors that explain some of the observed disconnect between neuropathology and clinical dementia. More specifically, through integration of in vivo biomaker and autopsy data into a unified model of resilience, we are able to perform large, comprehensive analyses of genetic resilience.
-
Defining Resilience
Quantifying resilience is challenging. Past research was often limited to individuals with “asymptomatic” Alzheimer’s disease (AD), a phenomenon in which individuals show no memory loss or other symptoms of AD during life, but after death their brains show all the signs of this disease (e.g., β-amyloid plaques and aggregation of tau). However, this definition of resilience drastically compromises statistical power by focusing on only a small subset of the population. Adding to the challenge, resilience definitions often combine the construct of reserve (e.g., predisposition towards protection) with resilience (e.g., better than expected performance despite neuropathology).
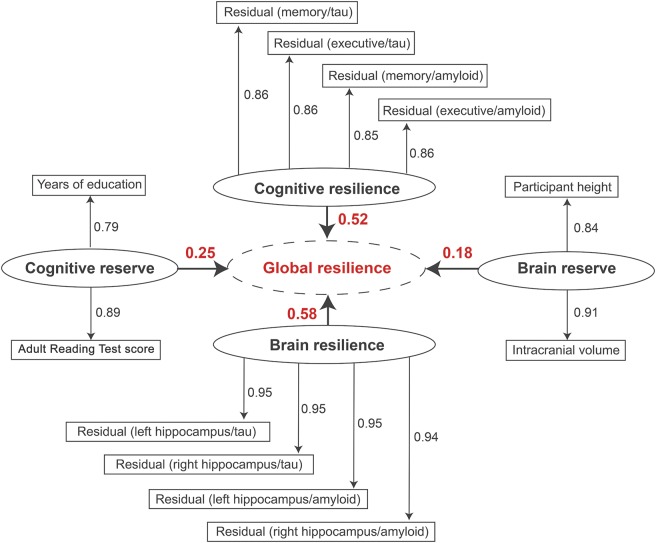
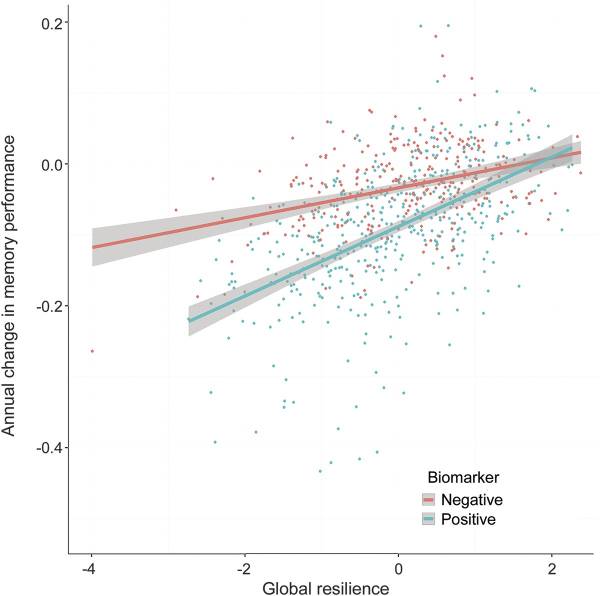
Our work addresses these challenges by leveraging a continuous resilience measure. Response to pathology appears to be best represented on a continuum given that the vast majority of cognitively normal individuals have at least some neuropathology at autopsy, and there is substantial heterogeneity across people who meet pathological criteria for AD. Building on the cognitive and brain reserve literature, we integrated co-calibrated cognitive data along with AD biomarkers to construct composite resilience measures (Figure 1). Our composite resilience measures can be harmonized across datasets, strongly predict protection from cognitive impairment (Figure 2), are easy to interpret, and enable large, well-powered resilience analyses.
Figure 1. Partial least squares (PLS) path model results. PLS path model results are presented; the goodness of fit was 0.76. Each first-order latent variable is presented as an oval. The variables included in each latent trait because we used reflective measurement. For the resilience metrics, each rectangle represents the residuals from a single linear regression model relating the given biomarker to the given outcome. The second-order latent variable (global resilience) is presented in a dotted oval. The loadings for each first-order latent variable are presented numerically above the bold arrows pointing to global resilience. (Hohman et al., Neurology, 2016)
Figure 2: Higher levels of global resilience are related to slower rates of cognitive decline, particularly among individuals who are positive for biomarkers of AD neuropathology. (Hohman et al., Neurology, 2016.)
-
Characterizing Drivers of Resilience
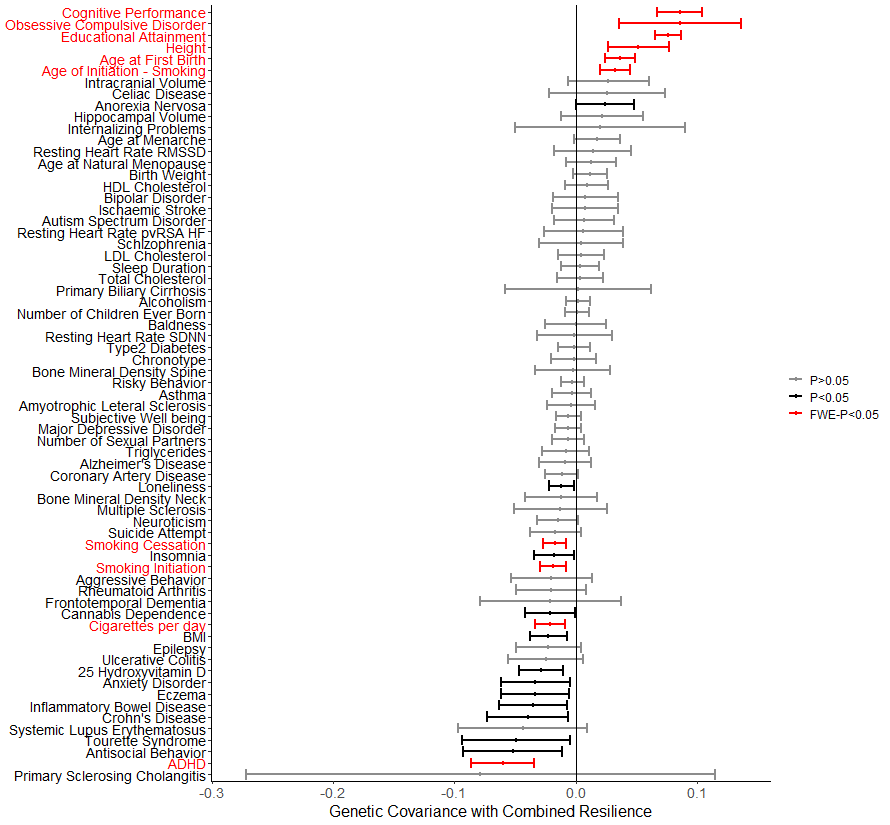
Identifying the molecular factors that underlie the resilience observed in asymptomatic AD may provide novel therapeutic targets for clinical intervention and provide additional insight into the genetic architecture of AD.
Robust phenotypes of resilience calculated by leveraging AD biomarkers and baseline brain aging outcomes provide insight into which individuals are at greatest risk of short-term decline. Such understanding of drivers of resilience are needed to further our understanding of the mechanisms that protect individuals from the clinical manifestation of AD dementia, especially among biomarker-positive individuals.
Figure 3: (Dumitrescu et al. 2020, Brain.)
-
Validating Drivers of Resilience
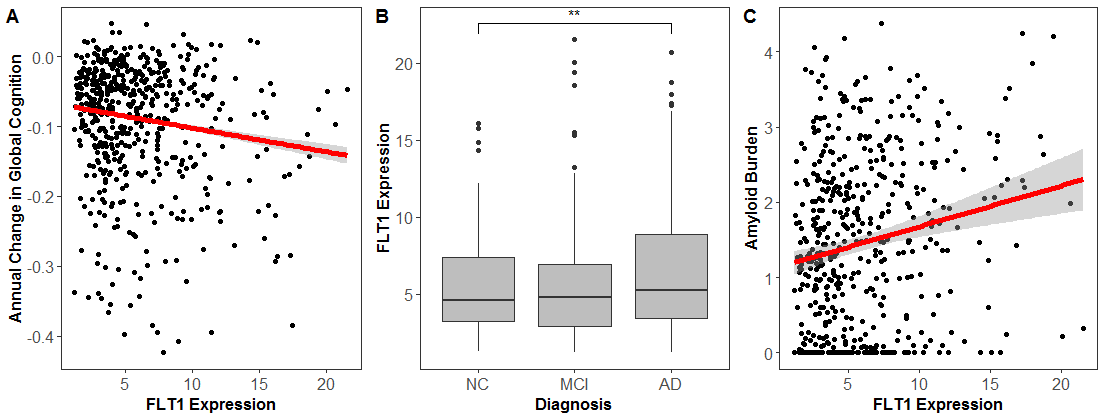
Robust Vascular endothelial growth factor (VEGF) is associated with the clinical manifestation of Alzheimer's disease (AD). However, the role of the VEGF gene family in neuroprotection is complex due to the number of biological pathways they regulate. Our studies in the associations between brain expression of VEGF genes with cognitive performance and AD pathology have found that VEGF ligand and receptor genes, specifically genes relevant to FLT4 and FLT1 receptor signaling, are associated with cognition, longitudinal cognitive decline, and AD neuropathology. Future work should confirm these observations at the protein level to better understand how changes in VEGF transcription and translation relate to neurodegenerative disease.
Figure 4: FLT1 expression associates with (A) longitudinal cognition, (B) clinical diagnosis, and (C) amyloid pathology. (Hohman et. al 2019, Molecular Psychiatry)
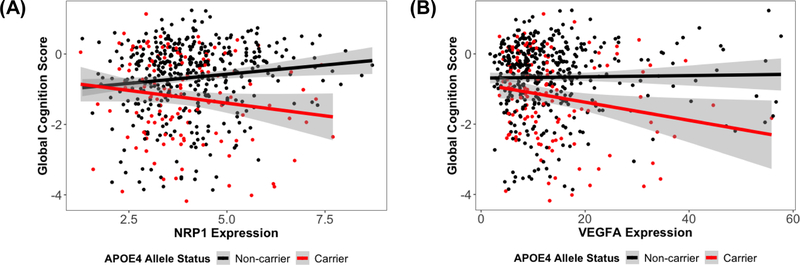
Figure 5: (A) NRP1 expression associations with global cognitive performance at the final neuropsychological assessment, stratified by APOE-ε4 allele status. Overall interaction:NRP1 × APOE-ε4, β=−0.28, p.fdr=0.007; APOE-ε4 carriers, β=−0.17, p=0.038; APOE-ε4 non-carriers, β=0.11, p=0.004. (B) VEGFA expression associations with global cognitive performance at the final neuropsychological assessment, stratified by APOE-ε4 allele status. Overall interaction: VEGFA × APOE-ε4, β=−0.03, p.fdr=0.026; APOE-ε4 carriers, β=−0.03, p=0.019; APOE-ε4 non-carriers, β=0.004, p=0.4. (Hohman et al. 2020, Neurobiology of Aging)
-
Dumitrescu L, Mahoney ER, Mukherjee S, Lee ML, Bush WS, Engelman CD, Lu Q, Fardo DW, Trittschuh EH, Mez J, Kaczorowski C, Hernandez Saucedo H, Widaman KF, Buckley R, Properzi M, Mormino E, Yang HS, Harrison T, Hedden T, Nho K, Andrews SJ, Tommet D, Hadad N, Sanders RE, Ruderfer DM, Gifford KA, Moore AM, Cambronero F, Zhong X, Raghavan NS, Vardarajan B, Pericak-Vance MA, Farrer LA, Wang LS, Cruchaga C, Schellenberg G, Cox NJ, Haines JL, Dirk Keene C, Saykin AJ, Larson EB, Sperling RA, Mayeux R, Bennett DA, Schneider JA, Crane PK, Jefferson AL, Hohman TJ. Genetic variants and functional pathways associated with resilience to Alzheimer's disease. Brain: A Journal of Neurology. 2020 Dec 1;143(143). 2561-2575.
PMCID: PMC7447518Moore AM, Mahoney E, Dumitrescu L, De Jager P, Koran MEI, Petyuk VA, Robinson RA, Ruderfer DM, Cox NJ, Schneider JA, Bennett DA, Jefferson AL, Hohman TJ. APOE ε4-specific associations of VEGF gene family expression with cognitive aging and Alzheimer's disease. Neurobiology of Aging. 2020 Dec;87(87). 18-25.
PMCID: PMC7064375Armstrong NM, Dumitrescu L, Huang CW, An Y, Tanaka T, Hernandez D, Doshi J, Erus G, Davatzikos C, Ferrucci L, Resnick SM, Hohman TJ. Association of hippocampal volume polygenic predictor score with baseline and change in brain volumes and cognition among cognitively healthy older adults. Neurobiology of Aging. 2020 Dec;94(94). 81-88.
PMID: 32593031 [PubMed]Archer DB, Moore EE, Shashikumar N, Dumitrescu L, Pechman KR, Landman BA, Gifford KA, Jefferson AL, Hohman TJ. Free-water metrics in medial temporal lobe white matter tract projections relate to longitudinal cognitive decline. Neurobiology of Aging. 2020 Dec;94(94). 15-23.
PMCID: PMC7483422Mahajan UV, Varma VR, Griswold ME, Blackshear CT, An Y, Oommen AM, Varma S, Troncoso JC, Pletnikova O, O'Brien R, Hohman TJ, Legido-Quigley C, Thambisetty M. Correction: Dysregulation of multiple metabolic networks related to brain transmethylation and polyamine pathways in Alzheimer disease: A targeted metabolomic and transcriptomic study. PLoS Medicine. 2020 Oct;17(17). e1003439.
PMCID: PMC7577459Raghavan NS, Dumitrescu L, Mormino E, Mahoney ER, Lee AJ, Gao Y, Bilgel M, Goldstein D, Harrison T, Engelman CD, Saykin AJ, Whelan CD, Liu JZ, Jagust W, Albert M, Johnson SC, Yang HS, Johnson K, Aisen P, Resnick SM, Sperling R, De Jager PL, Schneider J, Bennett DA, Schrag M, Vardarajan B, Hohman TJ, Mayeux R. Association Between Common Variants in RBFOX1, an RNA-Binding Protein, and Brain Amyloidosis in Early and Preclinical Alzheimer Disease. JAMA Neurology. 2020 Jun 22.
PMCID: PMC7309575Mahoney ER, Dumitrescu L, Seto M, Nudelman KNH, Buckley RF, Gifford KA, Saykin AJ, Jefferson AJ, Hohman TJ. Telomere length associations with cognition depend on Alzheimer's disease biomarkers. Alzheimer's & dementia (New York, N. Y.). 5(5). 883-890.
PMCID: PMC6926345Mahoney ER, Dumitrescu L, Moore AM, Cambronero FE, De Jager PL, Koran MEI, Petyuk VA, Robinson RAS, Goyal S, Schneider JA, Bennett DA, Jefferson AL, Hohman TJ. Brain expression of the vascular endothelial growth factor gene family in cognitive aging and alzheimer's disease. Molecular psychiatry. 2019 Jul 22.
PMCID: PMC6980445Hohman TJ, McLaren DG, Mormino EC, Gifford KA, Libon DJ, Jefferson AL. Asymptomatic Alzheimer disease: Defining resilience. Neurology. 2016 Dec 6;87(87). 2443-2450.
PMCID: PMC5177674Hohman TJ, Dumitrescu L, Cox NJ, Jefferson AL. Genetic resilience to amyloid related cognitive decline. Brain imaging and behavior. 2017 Dec;11(11). 401-409.
PMCID: PMC5392179Hohman TJ, Cooke-Bailey JN, Reitz C, Jun G, Naj A, Beecham GW, Liu Z, Carney RM, Vance JM, Cuccaro ML, Rajbhandary R, Vardarajan BN, Wang LS, Valladares O, Lin CF, Larson EB, Graff-Radford NR, Evans D, De Jager PL, Crane PK, Buxbaum JD, Murrell JR, Raj T, Ertekin-Taner N, Logue MW, Baldwin CT, Green RC, Barnes LL, Cantwell LB, Fallin MD, Go RC, Griffith P, Obisesan TO, Manly JJ, Lunetta KL, Kamboh MI, Lopez OL, Bennett DA, Hardy J, Hendrie HC, Hall KS, Goate AM, Lang R, Byrd GS, Kukull WA, Foroud TM, Farrer LA, Martin ER, Pericak-Vance MA, Schellenberg GD, Mayeux R, Haines JL, Thornton-Wells TA. Global and local ancestry in African-Americans: Implications for Alzheimer's disease risk. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2016 Mar;12(12). 233-43.
PMCID: PMC4681680Hohman TJ, Bush WS, Jiang L, Brown-Gentry KD, Torstenson ES, Dudek SM, Mukherjee S, Naj A, Kunkle BW, Ritchie MD, Martin ER, Schellenberg GD, Mayeux R, Farrer LA, Pericak-Vance MA, Haines JL, Thornton-Wells TA. Discovery of gene-gene interactions across multiple independent data sets of late onset Alzheimer disease from the Alzheimer Disease Genetics Consortium. Neurobiology of aging. 2016 Feb;38(38). 141-150.
PMCID: PMC4735733
-
Collaborators
- Logan Dumitrescu, MS, PhD
Research Assistant Professor at Vanderbilt University Medical Center
- Angela Jefferson, PhD
Professor of Neurology at Vanderbilt University Medical Center
- Katherine Gifford, PsyD
Assistant Professor of Neurology at Vanderbilt University Medical Center
- Reña A. S. Robinson, PhD
Associate Professor of Chemistry at Vanderbilt University Medical Center
- Catherine Kaczorowski, PhD
Assistant Professor of Medicine at Tufts University; Evnin Family Chair in Alzheimer’s Research, Jackson Laboratories
- Paul Crane, MD, MPH
Professor, UW Depatment of Medicine; Psychometrics Component Lead, Clinical Core, ADRC
- Vladislav Petyuk, PhD
Data Scientist at Pacific Northwest National Laboratory
- William S. Bush, PhD
Associate Professor at Case Western Reserve University
- The Alzheimer’s Disease Genetics Consortium
- The Alzheimer’s Disease Sequencing Project
- Religious Orders Study and the Memory and Aging Project (ROS/MAP)
- Accelerating Medicines Partnership – Alzheimer’s Disease (AMP-AD)
- Logan Dumitrescu, MS, PhD