Lena Feleke is a top 40 finalist in Regeneron Science Talent Search...
Lena Feleke, a student from the School for Science and Math at Vanderbilt’s (SSMV) senior class, also a student at Martin Luther King Jr. Magnet High School, has been named as Tennessee’s only Top 40 Finalist in this year’s Regeneron Science Talent Search (https://www.societyforscience.org/regeneron-sts/). This national competition recognizes and empowers the most promising young scientists in the U.S. who are creating the ideas and solutions that solve our most urgent challenges.
Lena completed a project titled, “Assessing the Effects of Transcriptional and Post-Transcriptional Regulatory Elements on SLC3A1 Transgene Expression for Type A Cystinuria Gene Therapy.” Her research was focused on examining and testing variations in a gene that could be used in new treatments for a certain type of chronic kidney disease. Lena’s work was done in the laboratory of Matthew Wilson, MD, PhD, in Vanderbilt Medical Center’s Department of Medicine, Division of Nephrology and Hypertension. Lena’s direct mentor was Jennifer Peek, an MD-PhD student in Vanderbilt’s Medical Scientist Training Program who graduated herself from the SSMV in 2014. It was a wonderful full-circle moment for Ms. Peek to mentor a student herself after her career path was inspired by her SSMV research laboratory experience years ago.
Congrats to Lena and Jen!
Cell and gene therapy for kidney disease...
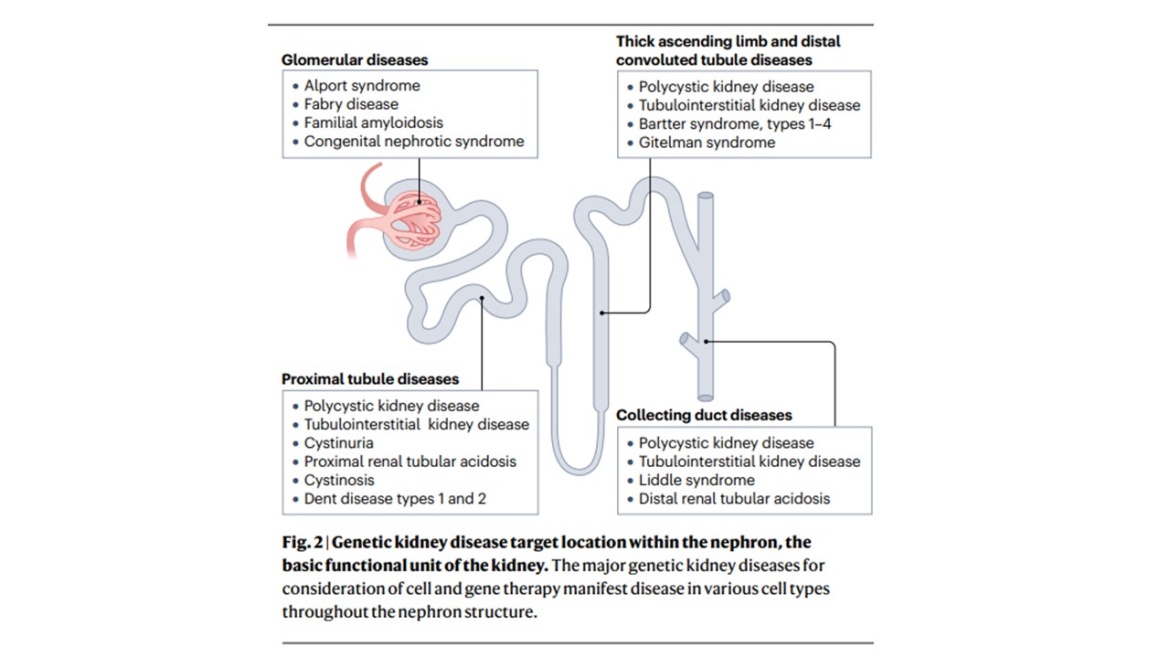
Kidney disease is a leading cause of morbidity and mortality across the globe. Current interventions for kidney disease include dialysis and renal transplantation, which have limited eficacy or availability and are often associated with complications such as cardiovascular disease and immunosuppression. There is therefore a pressing need for novel therapies for kidney disease. Notably, as many as 30% of kidney disease cases are caused by monogenic disease and are thus potentially amenable to genetic medicine, such as cell and gene therapy. Systemic disease that affects the kidney, such as diabetes and hypertension, might also be targetable by cell and gene therapy. However, although there are now several approved gene and cell therapies for inherited diseases that affect other organs, none targets the kidney. Promising recent advances in cell and gene therapy have been made, including in the kidney research field, suggesting that this form of therapy might represent a potential solution for kidney disease in the future. In this Review, we describe the potential for cell and gene therapy in treating kidney disease, focusing on recent genetic studies, key advances and emerging technologies, and we describe several crucial considerations for renal genetic and cell therapies.
Full article...https://doi.org/10.1038/s41581-023-00702-3
Transposase N-terminal phosphorylation and asymmetric transposon ends inhibit piggyBac transposition in mammalian cells
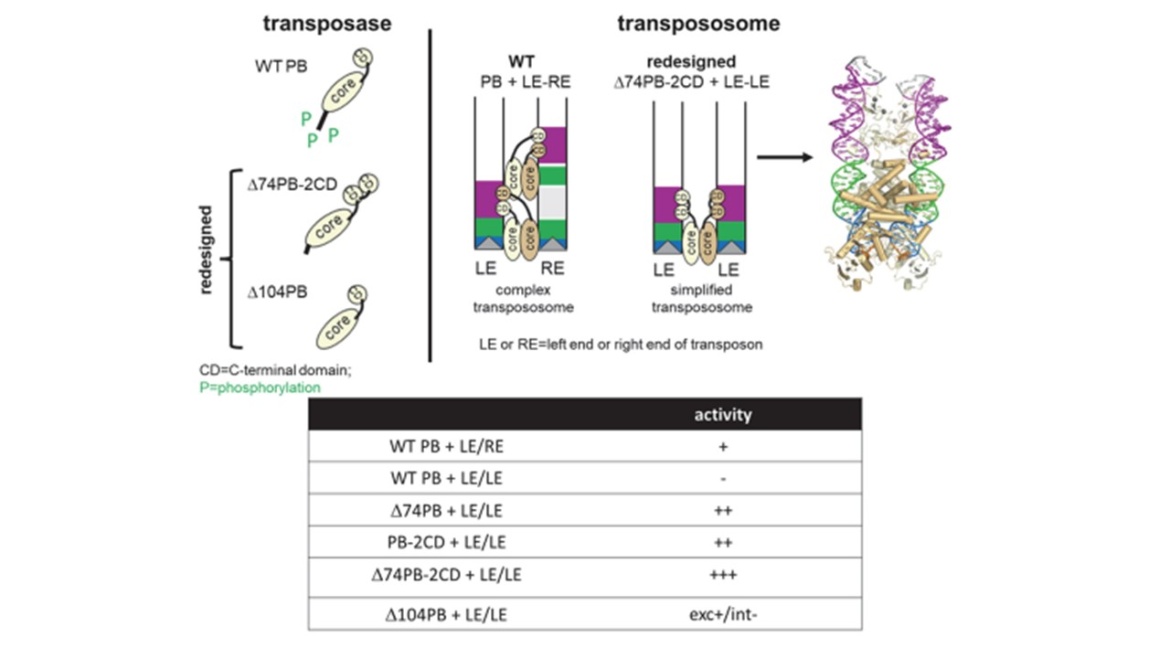
DNA transposon systems are widely used in mammalian cells for genetic modification experiments, but their regulation remains poorly understood. We used biochemical and cell-based assays together with AlphaFold modeling and rational protein re-design to evaluate aspects of piggyBac transposition including the previously unexplained role of the transposase N-terminus and the need for asymmetric transposon ends for cellular activity. We found that phosphorylation at predicted casein kinase II sites in the transposase N-terminus inhibits transposition, most likely by preventing transposase–DNA interactions. Deletion of the region containing these sites releases inhibition thereby enhancing activity. We also found that the N-terminal domain promotes transposase dimerization in the absence of transposon DNA. When the N-terminus is deleted, the transposase gains the ability to carry out transposition using symmetric transposon left ends. This novel activity is also conferred by appending a second C-terminal domain. When combined, these modifications together result in a transposase that is highly active when symmetric transposon ends are used. Our results demonstrate that transposase N-terminal phosphorylation and the requirement for asymmetric transposon ends both negatively regulate piggyBac transposition in mammalian cells. These novel insights into the mechanism and structure of the piggyBac transposase expand its potential use for genomic applications
Full article...https://doi.org/10.1093/nar/gkac1191