A COVID-19 Vaccine: Why We Need It and How We Get There Yesterday
The pandemic incited by the novel SARS-CoV-2 Virus has spread from a small outbreak in Wuhan, China to cover the entire world. The highly infectious nature of the virus, airborne transmission, and the ability of asymptomatic carriers to transmit the virus has led to the rapid spread of COVID-19 leading to over 62 million positive cases of infection from the virus worldwide with over 1.4 million deaths and counting. The United States has been hit harder than many other countries; with only 4.25% of the world’s population, the United States has recorded 20% (>13 million) of the total positive cases and deaths (>265,000).
Today’s COVID-19 outbreak is the largest seen in the world since the 1918 outbreak of the H1N1 virus, colloquially called the “Spanish Flu”, which lasted from April of 1918 to 1920, infecting one third of the word population (500 million individuals) and killing an estimated 50 million. As in 1918, social distancing and wearing masks are critical to reducing the spread of the virus; however, today we have the crucial need and the technology to develop a vaccine to more effectively prevent the spread of the virus and save countless human lives.
A group of pro-mask activists in California during the 1918 “Spanish Flu” Pandemic.
Adding some color helps us relate to a not-so-distant past.
Vintage Space/Alamy - JusxtaposeJS
A group of pro-mask activists in California during the 1918 “Spanish Flu” Pandemic.
Adding some color helps us relate to a not-so-distant past.
Vintage Space/Alamy - JusxtaposeJS
Outbreak Origins
At the time of the outbreak, there was no vaccine available or in development for the COVID-19 virus. The SARS-CoV-2 virus originated from a family of SARS-CoV viruses, which are commonly found in animals but are rarely able to bridge species and infect humans. It was first believed the virus was transmitted from bats to humans at a wet market in Wuhan where exotic animals are killed and prepared on-site. The Chinese Center for Disease Control and Prevention has since ruled out wet markets as the cause of the spread citing an inability to link animals at the market to coronavirus through testing, leading them to believe that the market actually served as a super spreader event. Debate is ongoing as to how the novel coronavirus infected humans, but it is believed through genetic evidence to have originated in Chinese bats and spread to humans through a yet unknown intermediary species.
Herd Immunity
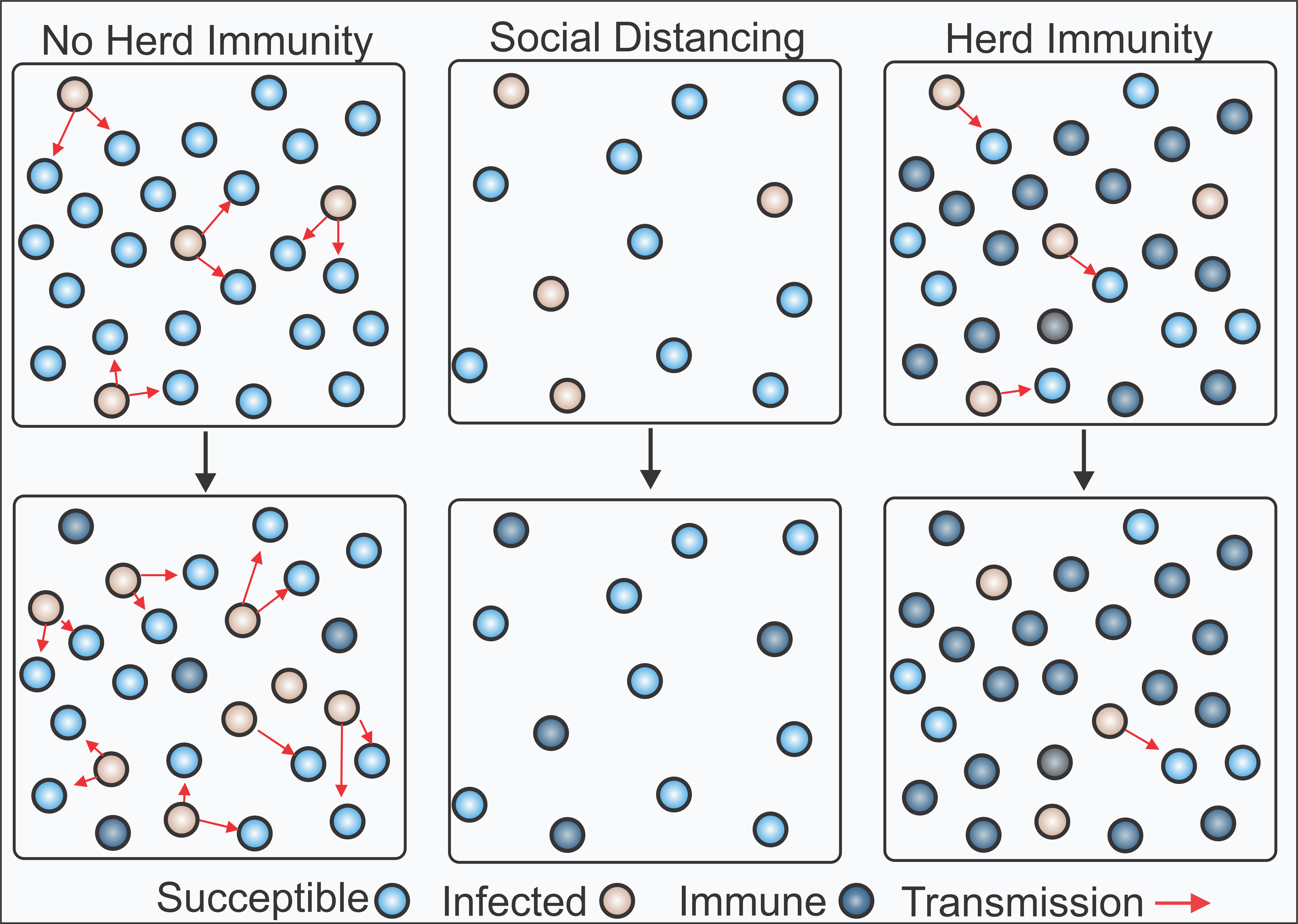
The novelty of this virus coupled with its high transmissibility and lethality has left the world scrambling to control the spread without overwhelming hospitals until we reach herd immunity.
After being infected and developing antibodies to overcome a virus, recovered individuals within a community become immune to further infection and are no longer able to carry or transmit a pathogen. Herd immunity is achieved once a certain percentage of the population develops immunity and becomes resistant to infection, limiting further spread of the pathogen. The percent of the population needed to reach herd immunity is unique to each pathogen and highly dependent on its infectiousness, described by the reproductive number R0, or how many persons an infected individual goes on to infect.
Reporting by the Wall Street Journal suggests that 60-70% of the population would need to develop resistance to COVID-19 before beginning to effectively limit the spread of the virus although, importantly, these calculations assume an equal susceptibility of all individuals to the virus which could overestimate the true values. Other projections estimate herd immunity to occur only at 90% resistance. The director of the Center for Disease Control (CDC) has stated that roughly 90% of American are still susceptible to the Corona Virus which is far from what is required for herd immunity. This susceptibility varies widely across the US with only 1% of the population in some areas having been infected by the virus.
After being infected and developing antibodies to overcome a virus, recovered individuals within a community become immune to further infection and are no longer able to carry or transmit a pathogen. Herd immunity is achieved once a certain percentage of the population develops immunity and becomes resistant to infection, limiting further spread of the pathogen. The percent of the population needed to reach herd immunity is unique to each pathogen and highly dependent on its infectiousness, described by the reproductive number R0, or how many persons an infected individual goes on to infect.
Reporting by the Wall Street Journal suggests that 60-70% of the population would need to develop resistance to COVID-19 before beginning to effectively limit the spread of the virus although, importantly, these calculations assume an equal susceptibility of all individuals to the virus which could overestimate the true values. Other projections estimate herd immunity to occur only at 90% resistance. The director of the Center for Disease Control (CDC) has stated that roughly 90% of American are still susceptible to the Corona Virus which is far from what is required for herd immunity. This susceptibility varies widely across the US with only 1% of the population in some areas having been infected by the virus.

Currently, social distancing measures recommended by the CDC have slowed the spread of the virus, but have also weakened the economy and significantly disrupted peoples’ lives. Achieving herd immunity is an important step in making it safe to return to normal life. Multiple solutions have been suggested, but the major challenge is reaching herd immunity without causing undue strain on our healthcare system and avoiding excessive deaths. As it stands, we have two options: achieve herd immunity through mass vaccination or controlling the natural spread.
Models to Control the Spread
In the early days of the pandemic, strategies of suppression, which involve control measures to inhibit the spread for a sustained period of time to eliminate the virus (i.e. lockdowns), or mitigation, which aims to lessen the negative impact of the virus by reducing its rate of transmission (i.e. social distancing, mask wearing), were considered. Due to the rapid spread of COVID-19 and the United States’ delayed response to the Corona Virus Pandemic, we are currently in a state of mitigation. Researchers argue, however, that continuing to control the spread through mitigation is not feasible.
In new research published in the Proceedings of the National Academy of Sciences (PNAS), Tobias Brett and Pejman Rohani of the ODUM School of Ecology at the University of Georgia suggest that while the COVID-19 virus could be suppressed by mitigation and control of social distancing practices, it “did not support achieving herd immunity as a practical objective, requiring an unlikely balancing of multiple poorly defined forces.”
Without herd immunity, the United States faces months more of social distancing and other mitigating measures to successfully repress the virus. In addition, there is the threat of COVID-19 resurgence, bringing the United States back to preventative measures, overwhelming hospitals, and causing excessive deaths. A vaccine for COVID-19 is critical to increasing the rate at which the United States can safely reach levels of herd immunity, suppress the virus, and return to a sense of normalcy.
Vaccines
Our bodies are continually fighting off invading viral, fungal, and bacterial pathogens to keep us healthy. After encountering a pathogen, our immune system learns to identify, neutralize, and remove the unwanted invader. A vaccine is a biologic treatment that teaches the immune system to respond so the body is ready to defend itself when it encounters a pathogen. The word vaccine derives from vaccinia, or the cow pox virus. In the late 1700s, a British doctor, Dr. Edward Jenner, infected several patients with cox pox and demonstrated these individuals were immune to infection by smallpox.
Today, vaccines can protect us without making us sick because they are made of either weakened forms of the pathogen or specific pieces of the pathogen, called antigens, in order to generate an immune response. After immunization, individuals are immune to infection for many years or even life. This is different than an antibody therapy, such as Regeneron’s Bamlanivimab which was recently granted emergency use authorization by the FDA for the treatment of COVID-19, which uses antibodies to neutralize a pathogen, offering protection for only weeks to months. For more information on this important distinction, check out this infographic by the VI4.
In the United States, it is recommended that children receive at least 14 vaccines by adolescence which has reduced the transmission of several common infections including polio, measles, mumps, rubella, and more recently the Human Papilloma Virus (HPV). Vaccination can even protect those who may not be able to be vaccinated for medical or religious reasons, by limiting the ability of a pathogen to spread through herd, or community, immunity.
Vaccine Development
Vaccines are typically developed through a lengthy, rigorous process that averages 10-15 years from inception to approval. The goal is to create a vaccine that is safe and with good immunogenicity, meaning it stimulates a strong immune response, to generate antibodies which can neutralize an invading pathogen. Multiple successive basic science, pre-clinical, and clinical stages must be completed sequentially before approval and distribution.
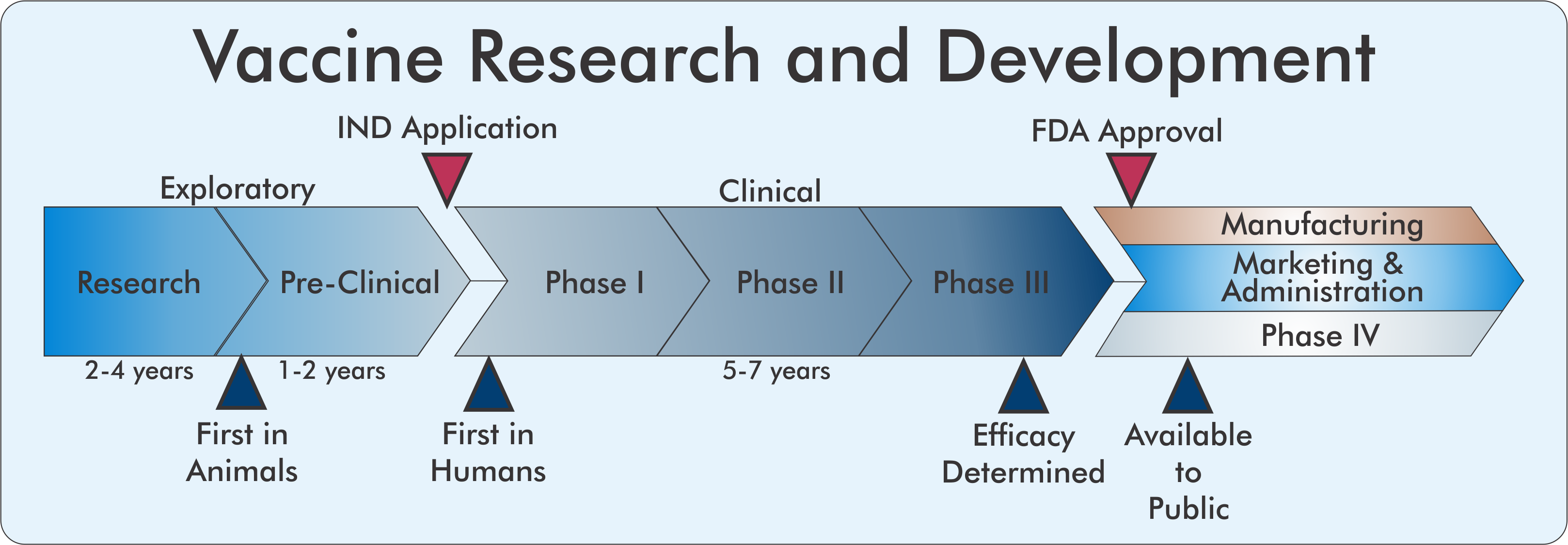
Exploratory Phase
Before a vaccine candidate reaches human subjects, academic labs conduct exploratory research to characterize a pathogen and determine what antigen, or piece of a pathogen, could stimulate a strong immune response. In the case of COVID-19, the Crowe lab of the VI4 and Vanderbilt University Medical Center has identified hundreds of antibodies within human coronavirus survivors that can neutralize SARS-CoV-2 by binding to a particular viral membrane protein called Spike. These findings suggest that the Spike protein could be an efficacious antigen to generate an immune response.
However, prior to using any biologically derived compounds in humans, extensive pre-clinical testing in animal models is required. Here, researchers can gain insight into the safety and immunogenicity of a vaccine by vaccinating animals and subsequently challenging them with a pathogen. Importantly, at this stage, the failure rate is extremely high because antigens that may have appeared promising in cell models often fail to generate the desired immune response in animals.
If a vaccine candidate has demonstrated safety and efficacy in animals, that does not guarantee success in human subjects. Vaccine development follows the three-phase clinical study structure to ensure the safety of human participants while determining its efficacy. Before entering clinical trials and dosing human subjects, a company must submit an Investigation New Drug (IND) application describing the nature of the vaccine, its production, safety and immunogenicity information, and clinical trial layout for approval by the Federal Drug Administration (FDA).
Clinical Phase
Phase I studies are the smallest and shortest of the clinical studies involving treatment of less than a hundred participants in order to ensure its safety. A successful candidate progresses to Phase II studies where hundreds of participants are dosed in a controlled fashion to determine an indication of the immunogenicity and efficacy of the vaccine, to determine the effective dose, and route of administration. Phase III is the largest and most costly study, typically involving thousands to tens of thousands of participants. These studies compare the treatment under study to either a placebo treatment or current standard-of-care and are typically either single- or double blinded, meaning either the participant alone or both the participant and the physician are blinded to whether the patient is receiving the vaccine or a placebo to prevent biasing the outcome of the study. Due to the scale of these studies, researchers can more precisely determine the efficacy and safety profile of the vaccine including identifying adverse events that may occur in a small percentage of the population.
Approval
A successful clinical stage ends with a detailed filing of the trial data to the FDA for review where regulators conduct a risk/benefit assessment to determine whether the drug can be marketed in the United States. Due to the high possibility of failure in late clinical stages, large scale manufacturing of a vaccine wouldn’t begin until late in the Phase III trials, after a strong indication of the vaccine’s efficacy can be determined.
Even after the extensive years of clinical testing, it is impossible for researchers to determine all possible adverse events that could occur in response to vaccine administration. Because of this, vaccines undergo an additional Phase IV study after the vaccine is approved for distribution to the general population. Adverse events can be reported to the Vaccine Adverse Event Reporting System (VAERS) which the CDC can use to determine safety after the product is launched.
Accelerated R&D
Given the high infectivity of COVID-19, the vast percentage of the population susceptible to the virus, and the burden it places on the healthcare system, the economy, and our lives, we need a vaccine much sooner than the normal 10-15-year timeline. To achieve a safe and effective vaccine within a reasonable time frame, a massive research effort within academia and the pharmaceutical industry must be undertaken in collaboration with and funded by the United States government.
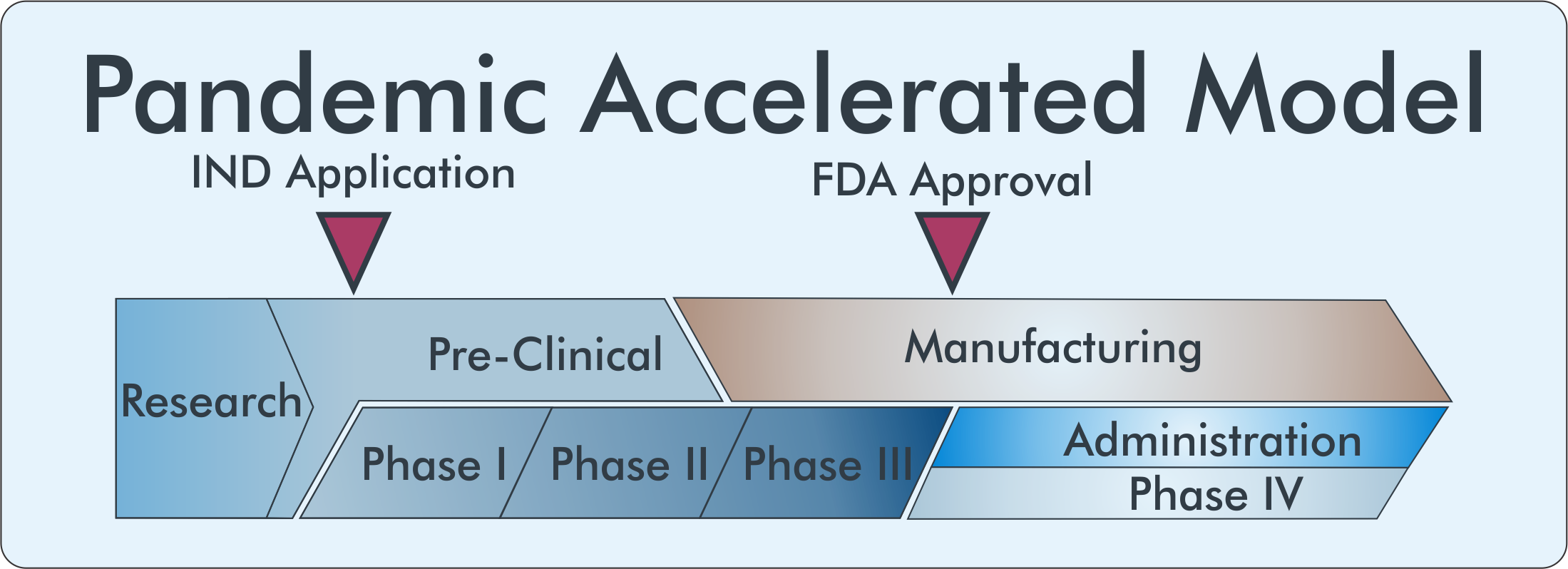
Accelerated R&D
Given the high infectivity of COVID-19, the vast percentage of the population susceptible to the virus, and the burden it places on the healthcare system, the economy, and our lives, we need a vaccine much sooner than the normal 10-15-year timeline. To achieve a safe and effective vaccine within a reasonable time frame, a massive research effort within academia and the pharmaceutical industry must be undertaken in collaboration with and funded by the United States government.

Funding
In response to the COVID-19 pandemic the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) partnership was founded to coordinate public resources and billions of dollars in funds with private industry to “develop a coordinated research strategy for prioritizing and speeding development of the most promising treatments and vaccines” according to the National Institutes of Health. This partnership involves 8 government agencies, 20 private pharmaceutical companies, and 4 Non-Profits to fast-track 4 critical areas: Identification of preclinical treatments, acceleration of clinical treatments, improve clinical trial efficiency and effectiveness, and accelerate the evaluation of vaccine candidates.
A separate public-private partnership, Operation Warps Speed (OWS), was also started in order to coordinate separate NIH and Biomedical Advanced Research and Development Authority (BARDA) directives, eliminate inefficiencies, and provide additional funding with the goal to “produce and deliver 300 million doses of safe and effective vaccines with the initial doses available by January 2021.”
Funding from government and non-profit organizations is essential to generating a vaccine for COVID-19. The high failure rate of vaccine candidates makes it extremely costly for pharmaceutical companies to perform clinical trials, especially with a vaccine market entry probability of only 6%. The US government, through the ACTIV and OWS partnerships, works to fiscally incentivize pharmaceutical companies by de-risking their investments into the research and development of life-saving treatments. Though there is still no vaccine which has been approved in the U.S., the government has already invested billions of dollars in hundreds of millions of doses of candidate vaccines in the pipeline from Pfizer and BionTech, Astrazeneca, Novavax, Johnson and Johnson, and Moderna.
Trial Design
Financially de-risking the investment of vaccine development, however, is not enough to deliver a vaccine by January 2021. The structure and logistics of clinical trials had to drastically change. Normally extensive safety testing is performed in animal models before a vaccine candidate moves to human trials; In the pandemic model, these stages are performed in parallel rather than sequentially.
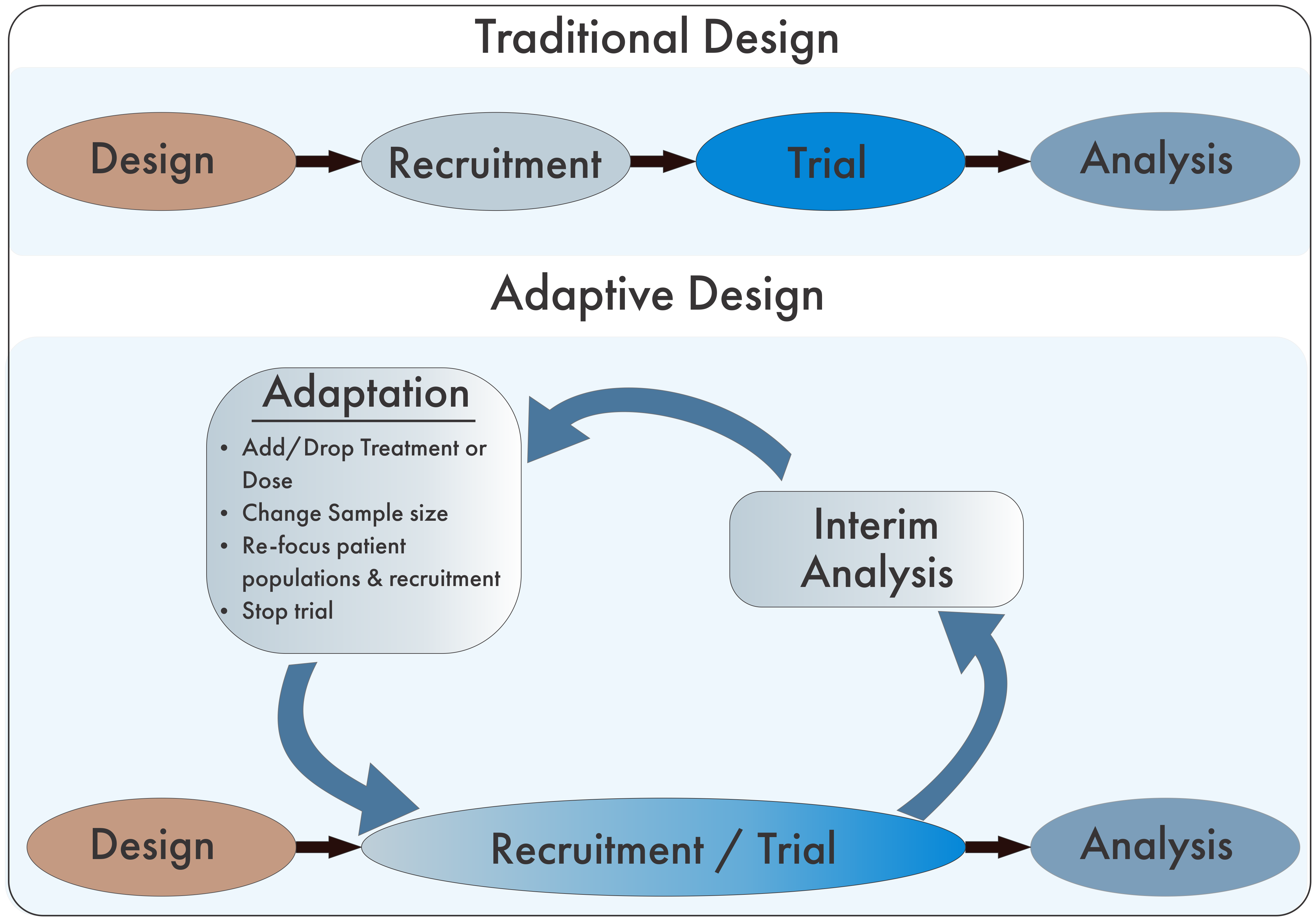
Adapted from Pallman P, et al. Adaptive designs in clinical trials: why use them, and how to run and report them. BMC Med. 2018;16:29.
In addition to the parallel nature of pre-clinical and clinical aspects of the development pipeline, the divisions between trial elements begin to blur as well. Phases can be combined into phase I/II or II/III trials allowing research questions to be answered more quickly and with fewer patients. The safety of human subjects is paramount and cannot be put aside in the accelerated model requiring FDA oversight to be expedited as well.
Adaptive Trials
Many companies are also switching from traditional fixed trial designs, in which pre-planned trials are conducted sequentially through stages of recruitment, conducting the trial, and final analysis, to an adaptive design. In the adaptive framework, data is consistently reviewed as it is being collected allowing for the trial to being consistently “adapted” to new information and the changing treatment landscape.
Multi-Arm-Multi-Stage Trials
In addition to the adaptive design, some trials have likewise adapted the Multi-Arm Multi-Stage (MAMS) trial design. Typical trials are randomized with patients within two treatment arms: the study treatment and either placebo or standard of care. While this design can function effectively, within the context of multiple competitors with concurrent development pipelines, companies can be pitted against each other for patient recruitment in this design. MAMS use multiple armed studies to simultaneously compare multiple treatments against one single control group. This design significantly decreases the numbers of patients that would be required for multiple two-armed trials while simultaneously increasing the number of patients who are receiving a potentially beneficial treatment.
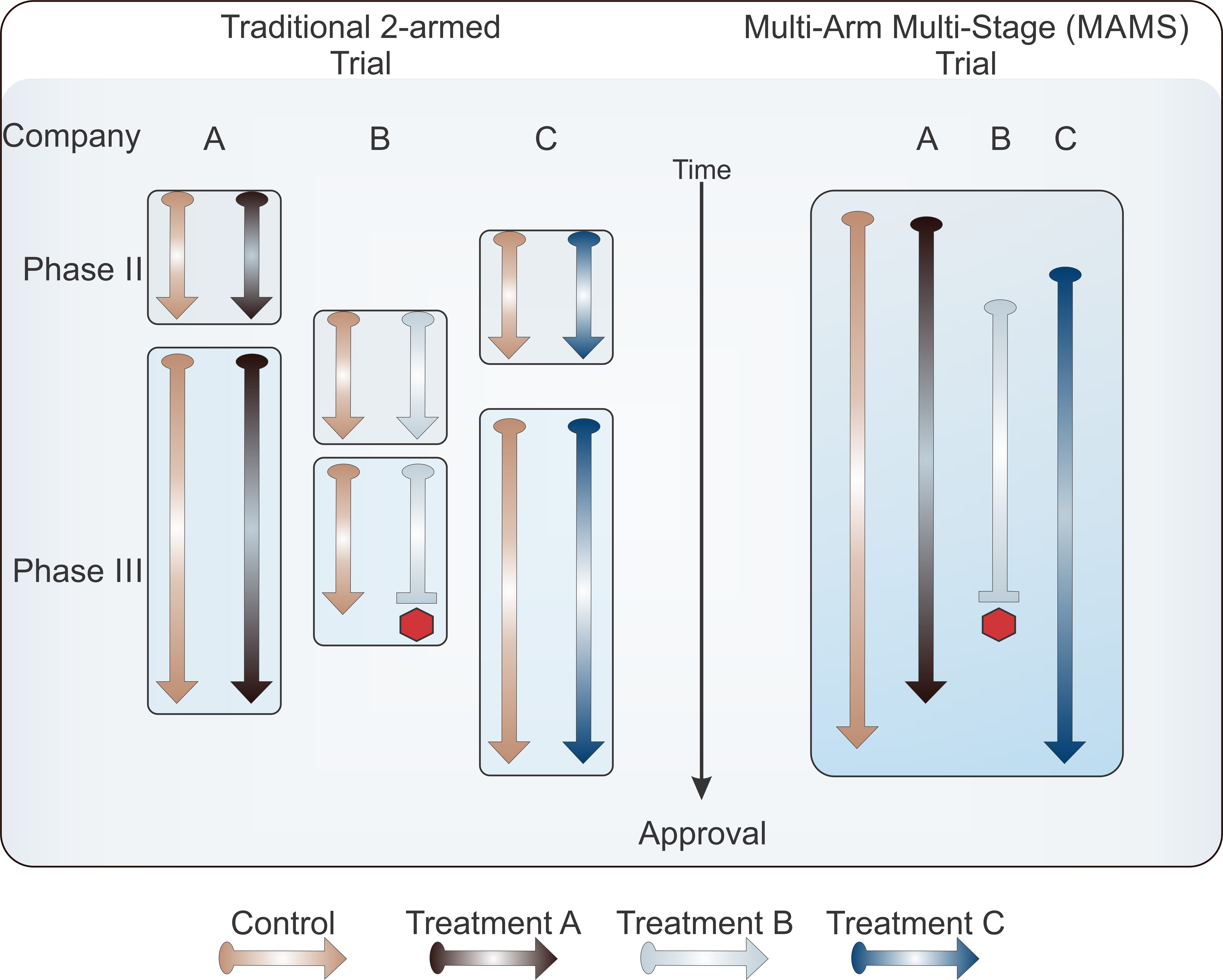

Adapted from Pallman P, et al. Adaptive designs in clinical trials: why use them, and how to run and report them. BMC Med. 2018;16:29.
In addition to the parallel nature of pre-clinical and clinical aspects of the development pipeline, the divisions between trial elements begin to blur as well. Phases can be combined into phase I/II or II/III trials allowing research questions to be answered more quickly and with fewer patients. The safety of human subjects is paramount and cannot be put aside in the accelerated model requiring FDA oversight to be expedited as well.
Adaptive Trials
Many companies are also switching from traditional fixed trial designs, in which pre-planned trials are conducted sequentially through stages of recruitment, conducting the trial, and final analysis, to an adaptive design. In the adaptive framework, data is consistently reviewed as it is being collected allowing for the trial to being consistently “adapted” to new information and the changing treatment landscape.
Multi-Arm-Multi-Stage Trials
In addition to the adaptive design, some trials have likewise adapted the Multi-Arm Multi-Stage (MAMS) trial design. Typical trials are randomized with patients within two treatment arms: the study treatment and either placebo or standard of care. While this design can function effectively, within the context of multiple competitors with concurrent development pipelines, companies can be pitted against each other for patient recruitment in this design. MAMS use multiple armed studies to simultaneously compare multiple treatments against one single control group. This design significantly decreases the numbers of patients that would be required for multiple two-armed trials while simultaneously increasing the number of patients who are receiving a potentially beneficial treatment.

The Word Health Organization began a multi-arm adaptive study for the production of Vaccine and drug candidates for the treatment of COVID-19 called the Solidarity Trial in April 2019. Within this trial structure, new treatment arms can be added as treatments are emerging, those that pose safety concerns can be paused or stopped, but the overarching trial continues, providing critical safety and efficacy data of the vaccine and drug treatments it’s testing.
A similar effort has been undertaken within the United States where the NIH partnership, ACTIV, has advised industry on trial designs to create harmonized protocols, to enable “analyses of correlates of protection across several vaccine trials being implemented under Operation Warp Speed.” Currently, vaccines from manufacturers Moderna, Astrazeneca, Sanofi and GlaxoSmithKline, and Johnson and Johnson are enrolled in the harmonized protocol with 3 of these 4 vaccines in phase III clinical trials. There are an additional 8 vaccines currently in Phase III trials outside of the harmonized protocol platform.
A vaccine on the horizon?
With 11 vaccines in phase III clinical trials, does that mean we’ll see a vaccine by 2020’s end? The answer to this question is not so simple. In November, Pfizer/Biontech and Moderna released the results of a completed phase III trial or interim analyses, respectively, showing roughly 94-95% efficacy of their COVID-19 vaccines, a number which surpassed the efficacy of some long established vaccines, beat expectations from experts, and far exceeded the minimum 50% efficacy required for approval from the FDA. Notably, Moderna’s vaccine had 100% efficacy in preventing severe COVID-19 cases; of 11 total cases observed, “All 11 cases occurred in the placebo group and none in the mRNA-1273 vaccinated group.” However, it may be too early to tell whether these vaccines will be a magic bullet to the pandemic.
Healthcare experts from Mckinsey and Company state, “Caution is still warranted. The safety records of the Pfizer and Moderna vaccines appear promising so far (no serious side effects reported), but the coming months will provide a fuller picture as the sample size grows.” Additionally, while these vaccines have proven efficacious in adult populations, it's going to take months longer for approval in children. Excluding children from the immunization efforts is projected to increase the percentage of the population requiring vaccination to reach herd immunity from 45-65% to 60-85% according to Mckinsey. An increased number of necessary doses can add strain to an already difficult rollout and distribution. This projection also relies on an assumption of vaccine-acquired immunity lasting 6-9 months, based on the lasting natural immunity of COVID-19 survivors. A lasting immunity to the virus by vaccination is critical to overcoming the virus but this duration of immunity has not yet been determined for the current vaccine candidates and can only be determined with time. As of November 30th, both Pfizer/Biontech and Moderna have filed for emergency use authorization from the FDA and expect to produce 50 million and 20 million vaccine doses, respectively, by the end of 2020. With each vaccine requiring two shots, these doses will immunize half these number of individuals.
More vaccines are entering trials or approaching approval with each passing day, however the possibility of phase III failures and delays still loom.
Diversity of Trial Populations
Enrollment for some vaccine trials has been slowed or even halted due to the low enrollment of minority and elderly participants, despite an increased risk for COVID-19 complications in these populations. To maximize the safety and efficacy of a treatment, the diversity of a trial population should mirror that of the population that will receive treatment; however, according to the FDA, in 2019 trial populations for approved drugs were 72% White, 9% Black or African American, 9% Asian, 18% Hispanic, and 36% age 65 and older. Outreach efforts have been aimed at recruiting minority groups while trial enrollment has slowed to leave room for more underserved populations.
Safety Delays
Even with sufficient and diverse enrollment, companies still face an uphill battle with trial delays. Recently, AstraZeneca had to delay its phase III studies after a patient developed a rare neurologic disorder during treatment; trials have since resumed. It is anticipated that by December of 2020, companies will have indications of the efficacy of their treatments but that doesn’t mean you’ll be getting vaccinated at your local treatment center by then; Final data analysis may not conclude until March 2021. After that, large scale manufacturing of approved vaccines, though already begun, will have to be expanded even further.
Production and Distribution
With a potential approval in early 2021, vaccines would be first distributed to those most susceptible to the virus, including healthcare workers, until manufacturing could match demand. Logistics of vaccine dispersal further complicate the matter; two of the top three vaccine candidates, made by AstraZeneca (AZD122) and Moderna (mRNA-1273), require two doses and refrigeration for transport. This increases the number of necessary manufactured doses for individuals complicating production and transport.
A candidate adenovirus vaccine from Johnson and Johnson (JNJ-78436725) recently entered Phase III clinical trials and has been met with high hopes from the healthcare community. The adenovirus vaccine platform has proven safe and efficacious in the past and importantly this vaccine requires only one injection and does not need to be frozen. Given the possibility for delay and failure in trials, manufacturing, and logistics, most experts, including the director of the CDC, expect to see a vaccine to the American public closer to mid-2021.
Public Trust
The development of a vaccine in a 1.5-year time frame is a truly herculean effort and a testament to the dedication of our hardworking public and private healthcare industries. One question that cannot be answered with a timeline though, is trust. Will the American people trust a vaccine produced within this limited time frame? Currently, polls suggest that only 50% of Americans plan on receiving a COVID-19 vaccine. According to a Gallup poll, 35% of Americans say they wouldn’t take a free, FDA-approved vaccine if it was available today. Given more time, with a vaccine available by mid-2021, will Americans get vaccinated, reach herd immunity, and suppress the spread of COVID-19? Only time will tell.
COVID-19 cases and information at the time of publication.

