SARS-CoV-2 evolution and implications for immunity
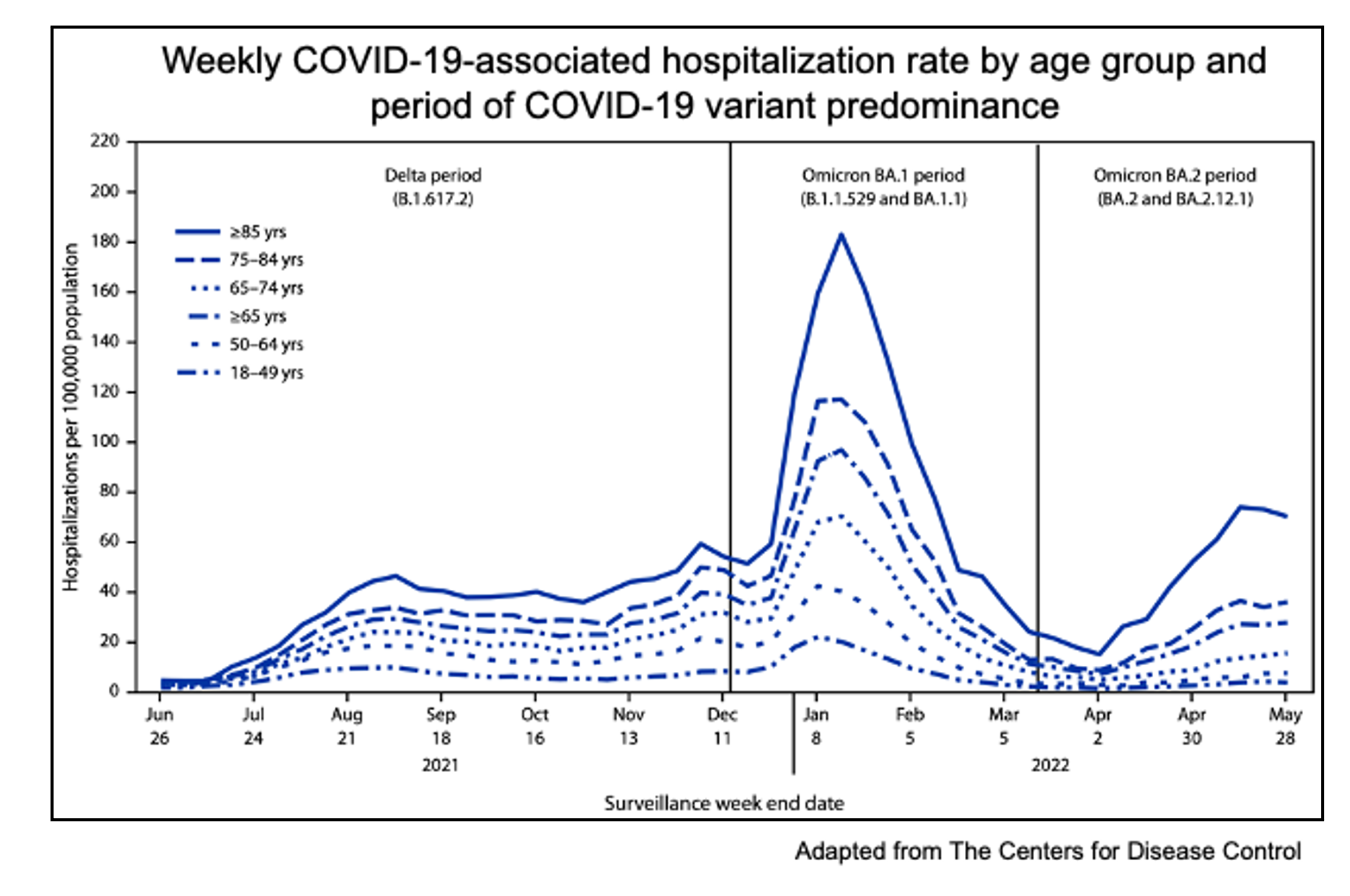
While COVID-19 is no longer plaguing media outlets, the virus remains a serious threat to human health. Since the discovery of SARS-CoV-2, the virus and its transmissibility evolved. The Omicron variant is the dominant circulating virus that causes COVID-19 in the United States and is capable of evading pre-existing immunity, causing serious public health concern. In fact, the greatest number of COVID-19 related hospitalizations occurred during the first Omicron wave. With high burdens of respiratory syncytial virus (RSV) and influenza this season, it is important to remain vigilant and take steps to prevent severe COVID-19 illness.
SARS-CoV-2 evolution has long-term implications on human health.
It remains unclear whether the intrinsic evolution of the SARS-CoV-2 virus or the ability of the Omicron variant to evade pre-existing immunity has resulted in rapid spreading of the virus in humans. Scientific studies have found that the Omicron variant is less pathogenic compared to the ancestral SARS-CoV-2 virus and is less capable of binding and fusing with human cells. The adaptations enable the virus to evade the immune response and survive in young and healthy hosts with potentially fewer symptoms. Because of this, individuals infected with COVID-19 unknowingly spread the virus to elderly individuals and others with compromised immune systems, resulting in severe and fatal cases of disease.
Healthy individuals should also consider the implications of Long COVID, which could include extenuated fatigue, exercise and walking intolerance, muscle pain, and shortness of breath. Long COVID can occur regardless of whether people exhibit signs of severe disease while infected with the virus. However, there is a correlation between the severity of illness and respiratory symptoms at the onset of disease and the development of Long COVID.
The COVID-19 virus currently circulating has mutations that facilitate infection.
The COVID-19 Omicron variant (B.1.1.529) was deemed the fifth variant of concern by the Centers for Disease Control and Prevention (CDC) in November of 2021. This COVID-19 variant has over 18,000 genomic mutations compared to the original SARS-Cov-2 virus, with more than thirty of these mutations falling within the spike protein receptor-binding domain (RBD). The seemingly rapid mutation rate of SARS-CoV-2 is not thought to differ dramatically from other coronaviruses.
“Unlike other coronaviruses that have been with us for a long time, SARS-CoV-2 entered a population of billions of immunologically naïve hosts,” explained Dr. Andrea Pruijssers, former Director of the Coronavirus Antivirals Program in the laboratory of Dr. Mark Denison.
Dr. Pruijssers stated that the lack of pre-existing immunity against SARS-CoV-2 increased the reservoirs for the virus to accumulate neutral and beneficial mutations. Ultimately, a lack of immunity in humans led to the rapid diversification of the SARS-CoV-2 genome and the emergence of variants.
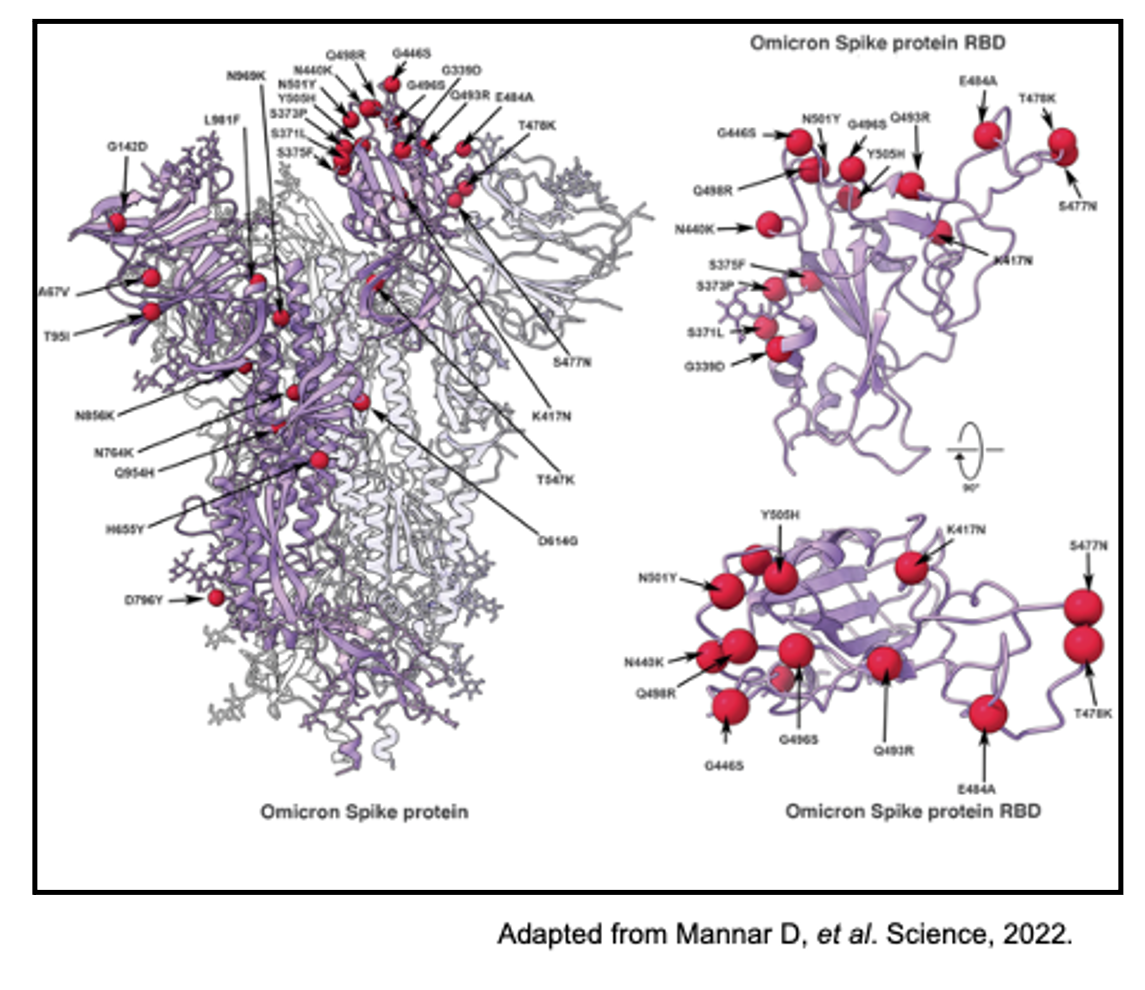
The COVID-19 Omicron variant (B.1.1.529) was deemed the fifth variant of concern by the Centers for Disease Control and Prevention (CDC) in November of 2021. This COVID-19 variant has over 18,000 genomic mutations compared to the original SARS-Cov-2 virus, with more than thirty of these mutations falling within the spike protein receptor-binding domain (RBD). The seemingly rapid mutation rate of SARS-CoV-2 is not thought to differ dramatically from other coronaviruses.

“Unlike other coronaviruses that have been with us for a long time, SARS-CoV-2 entered a population of billions of immunologically naïve hosts,” explained Dr. Andrea Pruijssers, former Director of the Coronavirus Antivirals Program in the laboratory of Dr. Mark Denison.
Dr. Pruijssers stated that the lack of pre-existing immunity against SARS-CoV-2 increased the reservoirs for the virus to accumulate neutral and beneficial mutations. Ultimately, a lack of immunity in humans led to the rapid diversification of the SARS-CoV-2 genome and the emergence of variants.
Prior SARS-CoV-2 variants contained RBDs that were dynamic, oscillating between conformations that allow for binding to the ACE2 receptor on human cells as well as a “closed” conformation that does not efficiently bind to ACE2. Neutralizing antibodies generated via prior vaccination target a cryptic epitope of the SARS-CoV-2 spike protein RBD that is present when the virus RBD is in a closed conformation.
Mutations within the Omicron variant RBD stabilize the spike protein on the virus in a confirmation that facilitates binding to ACE2. However, the stabilized spike conformation does not alter the variant’s affinity for the ACE2 receptor on human cells compared to the original SARS-CoV-2 virus, suggesting that these mutations primarily increase fitness by enhancing immune evasion.
In line with structural studies that identified the conformational change in the Omicron spike protein, analysis of human sera for neutralization activity against the Omicron variant decreased compared to prior variants. Human sera from double vaccinated individuals had a 20+ fold decreased neutralization activity against Omicron compared to prior variants after vaccination. The stabilization of the spike protein in a conformation that avoids antibody binding leads to the Omicron SARS-CoV-2 variant effectively increasing its chances of evading immune response from prior vaccination. Advances in vaccine design are required to combat mutations that render the Omicron variant capable of evading the previously mounted immune response.
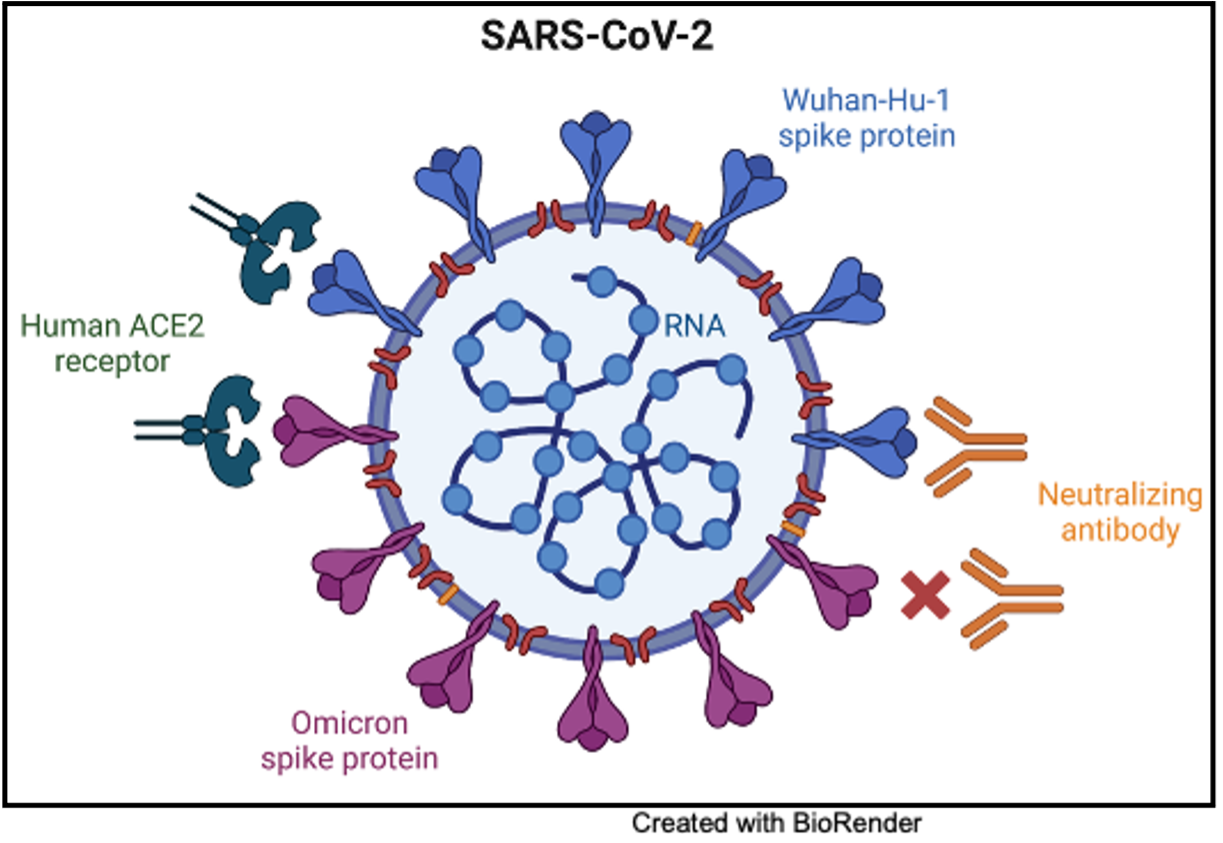
In line with structural studies that identified the conformational change in the Omicron spike protein, analysis of human sera for neutralization activity against the Omicron variant decreased compared to prior variants. Human sera from double vaccinated individuals had a 20+ fold decreased neutralization activity against Omicron compared to prior variants after vaccination. The stabilization of the spike protein in a conformation that avoids antibody binding leads to the Omicron SARS-CoV-2 variant effectively increasing its chances of evading immune response from prior vaccination. Advances in vaccine design are required to combat mutations that render the Omicron variant capable of evading the previously mounted immune response.


