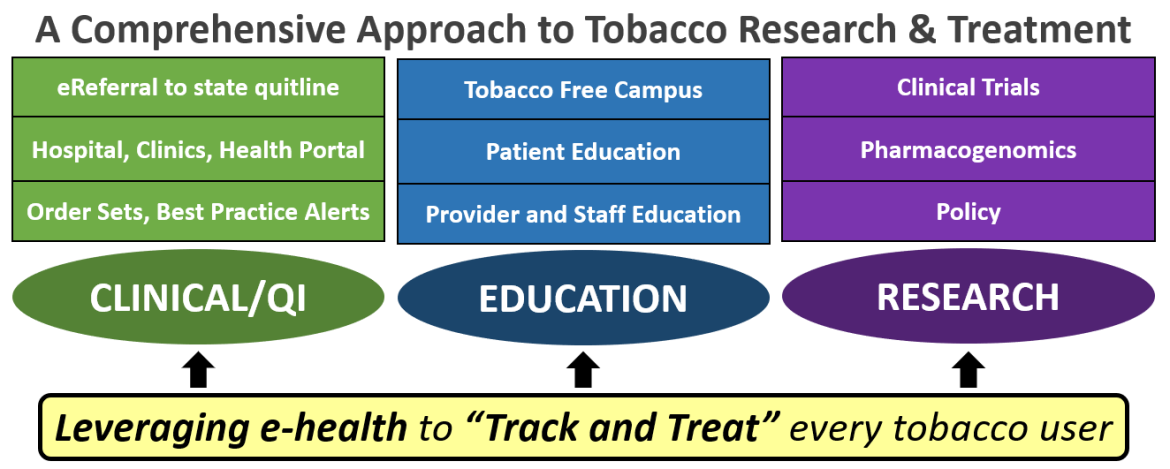
ViTAL, the Vanderbilt Center for Tobacco, Addiction and Lifestyle, was founded by Dr. Hilary Tindle, MD, MPH in 2014, with trans-institutional support aligning VUMC hospital operations, the VUMC Department of Medicine, the Vanderbilt-Ingram Cancer Center (VICC), and Divisions of General Internal Medicine & Public Health and Cardiology. To ensure a comprehensive approach to tobacco research and treatment across VUMC, the Center is comprised of three domains: clinical and quality improvement (QI), education, and research. Our research focuses on clinical trials for nicotine dependence and concomitant substance use, pharmacogenetics, and epidemiology.
-
ViTAL clinical and QI initiatives include the Tobacco Treatment Service (TTS), a clinical inpatient service staffed by Certified Tobacco Treatment Specialists (CTTS) that provide evidence-based care to tobacco users who are hospitalized. Founded by Dr. Tindle in 2015, the TTS provide opt-out care (i.e., direct consult is not required), which is becoming the accepted standard for tobacco treatment in clinical settings. The TTS assess and proactively visit patients identified from the Electronic Health Record (EHR) as current or former smokers (those who have quit within the past month and are at high risk of relapse). The CTTS assess patients’ tobacco use patterns, offer counseling, and recommend smoking cessation medications to manage nicotine withdrawal and promote long-term cessation. To achieve better long-term continuity of care, the TTS will utilize bi-directional electronic referral (“e-Referral”) to the TN state quitline. This project is modeled on a prototype led by Dr. Tindle during her tenure at the University of Pittsburgh Medical Center and has been adopted by the North American Quitline Consortium (NAQC) for the continent. All hospitalized smokers seen by the TTS are offered eReferral, and those who accept are contacted by the state quitline’s tobacco treatment specialists within 2 days of hospital discharge.
-
Dr. Tindle and ViTAL Center faculty member mentor trainees at the predoctoral, post-doctoral, and faculty level who are pursuing careers related to clinical research. In addition, ViTAL has launched educational campaigns across the medical center, including a VUMC-wide initiative to increase delivery of proven quit aids by hospital staff, including nurses and physicians. This campaign includes didactic lectures and leverages IT tools including a tobacco treatment order set and quick-reference ID badge cards to encourage greater prescribing of FDA approved smoking cessation medications and making referrals to the state quitline. ViTAL has spearheaded educational efforts related to new Joint Commission (JC) tobacco measures (now required by CMS) for the Vanderbilt Psychiatric Hospital. This quality improvement-driven clinical effort assists nursing staff, Addiction Psychiatry and Addiction Medicine postdoctoral fellows, and other providers to deliver and document care in order to satisfy the CMS requirements and improve care. TTS counselors serve as ambassadors for change across the clinical enterprise, including the adoption of system-wide improved methods to treat tobacco use and proactively reaching out to other staff, faculty, and trainees as appropriate in order to help demonstrate state-of-the-art tobacco treatment. Another example of serving as a Tobacco Control ambassador is fielding questions from faculty, staff, and trainees in an enthusiastic and scholarly manner, using up-to-date information from the medical literature to support treatment decisions and educate peers. These educational projects also offer excellent opportunities for trainees to engage in clinical research.
-
Dr. Tindle brings prodigious research experience to the ViTAL Center. She has authored many publications and currently serves as Principal Investigator or co-Principal Investigator of multiple NIH-funded comparative-effectiveness Randomized Control Trials for smoking cessation. These ongoing trials address smoking in unique populations: veterans of very low socioeconomic status, hospitalized smokers, and non-daily smokers. Historically, Dr. Tindle and members of the ViTAL Center have participated in a PI or MPI role in other clinical trials supported by NIDA, NIAAA, and NHLBI. These include productive collaborations with the Tennessee Center for AIDS Research (CFAR) to foster research advancing substance use treatment in people living with HIV (U01-AA020780). These trials, in combination with leveraging large datasets including the Framingham Heart Study (FHS), The Women’s Health Initiative (WHI), The Southern Community Cohort Study (SCCS) to name a few, provide a rich environment to support trainees in pursuit of their research questions.
