1. Evaluation of New Vaccines and Therapeutics
Vanderbilt investigators have expertise in a number of infectious diseases and specific, special populations.
Infectious Diseases of Interest
-
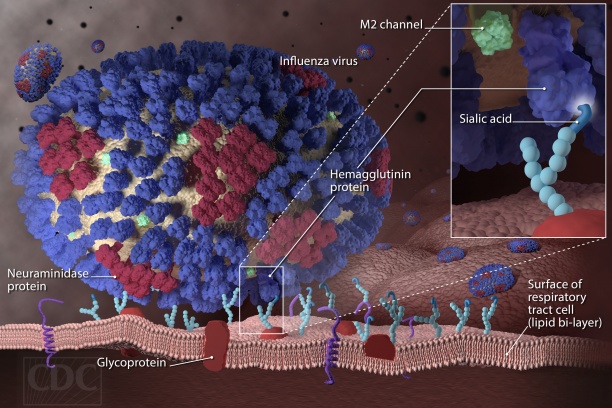
Influenza
-
Pertussis
-
S. aureus infections
-
SARS-CoV-2 (Coronavirus Disease)
-
Group B Streptococcus Infections
-
Clostridium difficile infections
-
Respiratory Syncitial Virus
-
Varicella Zoster Virus
-
Malaria
-
Mpox
2. Post-licensure Evaluation of Existing Agents
Once vaccines are licensed, the burden of disease often shifts considerably. Often, this leads to near-elimination of certain pathogens (like measles and polio), but sometimes can lead to unforeseen changes in the strains that cause disease (such as strain replacement that has been seen following pneumococcal vaccination). VVRP faculty are heavily engaged in disease surveillance in order to inform next generation vaccines and new vaccine strategies.
3. Investigations of Potential Adverse Events Following Immunization
Vaccines that are currently in use have excellent side effect profiles; however, no drug or vaccine can ever be completely harmless. Ongoing work in the VVRP includes evaluation of individual reports of adverse events following immunizations and evaluation of vaccines in patient populations that may be at higher risk of adverse events, such as immunocompromised hosts and those with asthma.
4. Deciphering the Host Immune Response to Infection and Immunization
Through campus-wide collaborations, VVRP investigators use cutting-edge technologies to assess immune responses in both healthy vaccinees and in those experiencing acute disease. Employing high-throughput sequencing, proteomics, and metabolomics, VVRP investigators are using systems biology tools to unlock basic mechanisms of human immune responses.