Nervous Systems Mechanisms in Bladder Dysfunction
This project examines how sensory and pain pathways, specifically central sensitization, impact on Overactive Bladder symptoms and treatment outcomes. The goal of this research is to identify a biomarker for sensory nerve dysfunction that can help distinguish individuals with OAB based on underlying pathophysiologic mechanisms. This project is funded by a two NIH grants: initially by a Career Development Award (K23DK103910 Afferent Hyperactivity Mechanisms in Overactive Bladder Syndrome) and continued most recently by a R01 grant (R01DK128293 Central Sensitization and Psychosocial Impacts on Overactive Bladder).
A recent expansion of this project involves studying these pathways for the first time using functional MRI of the spine.
Interested in participating or learning more? Click here to complete the screening survey or email Mariah to see if you qualify.
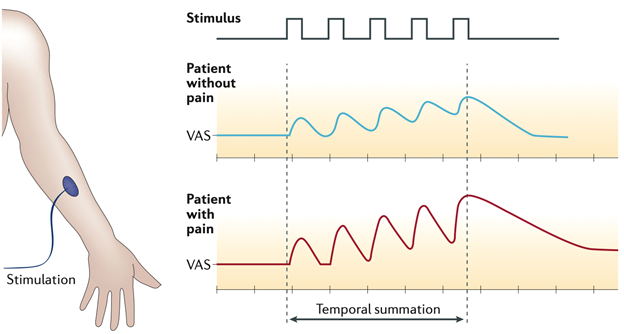
Figure 2 | Temporal summation. During quantitative sensory testing, the perception of pain intensity assessed with a visual analogue scale (VAS) in response to a repetitive thermal stimulation of uniform intensity applied to the forearm will gradually increase owing to central sensitization. In a patient with chronic pain, central sensitization facilitates temporal summation, whereas, in a healthy person, this intensity does not increase owing to habituation to the stimulus. From Reynolds et al, Nature Reviews Urology, 2016
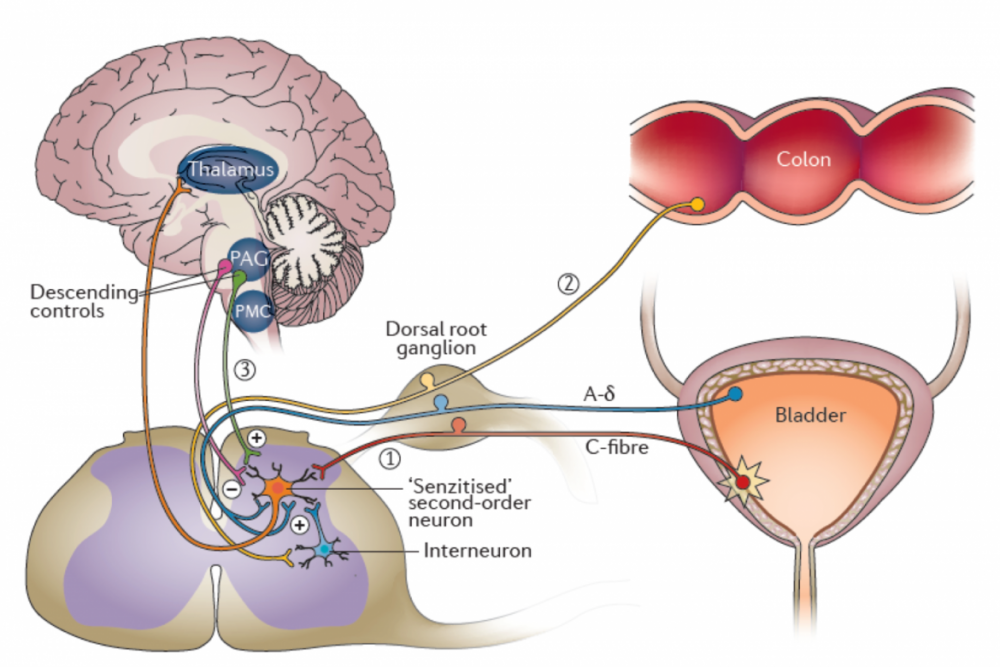
Figure 3 | Hypothetical roles of central sensitization in overactive bladder (OAB). Persistent activation of peripheral nociceptive C-fibers, such as those that project from the bladder or related pelvic organs (such as the colon), could induce central sensitization in second-order spinal neurons. Once established, central sensitization might contribute to overactive bladder by (1) facilitating ascending transmission of normally low-threshold mechanoreceptor signals from bladder afferents (afferent noise) or (2) from other pelvic organs via crosstalk with afferent signaling pathways that project from other organs. In addition (3), descending neural projections might also facilitate afferent spinal transmission of bladder signals in the setting of central sensitization. From Reynolds et al, Nature Reviews Urology, 2016.
Selected Publications
Reynolds WS, McKernan LC, Dmochowski RR, Bruehl S. The biopsychosocial impacts of anxiety on overactive bladder in women. Neurourol Urodyn. 2023 Feb 13. doi: 10.1002/nau.25152. PMID: 36780135
Reynolds WS, Dmochowski RR, Wein A, Bruehl S. Does central sensitization explain Idiopathic Overactive Bladder? Nature Reviews Urology. 2016, 13 (8): 481 – 491. PMID: 27245505, PMCID: PMC4969200.
Reynolds WS, Mock S, Zhang X, Kaufman MR, Wein A, Bruehl S, Dmochowski RR. Somatic syndromes and chronic pain in women with overactive bladder. Neurourology & Urodynamics. 2017; 36(4): 1113-1118. PMID: 27367486, PMCID: PMC5203978.
Reynolds WS, Brown ET, Danford JM, Kaufman MR, Wein A, Dmochowski RR, Bruehl S. Temporal summation to thermal stimuli is elevated in women with Overactive Bladder Syndrome. Neurourology & Urodynamics. 2017; 36(4):1108-1112. PMID: 27434229, PMCID: PMC5247414.
Reynolds WS, Kowalik CG, Cohn J, Kaufman MR, Wein A, Dmochowski RR, Bruehl S. (2018) Women undergoing third line overactive bladder treatment demonstrate elevated thermal temporal summation. Journal of Urology; 200(4):856-861. PMID: 29746857. PMCID: PMC6146049
Kowalik CG, Cohn J, Delpe S, Wein AJ, Kaufman MR, Dmochowski RR, Reynolds WS. (2018). Painful bladder symptoms related to somatic syndromes in a convenience sample of community women with overactive bladder symptoms. Journal of Urology; 201(1):129-134. PMID: 30017963. PMCID: PMC6298830.



