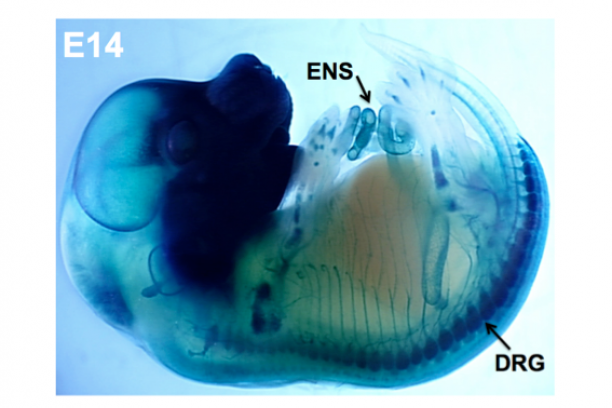
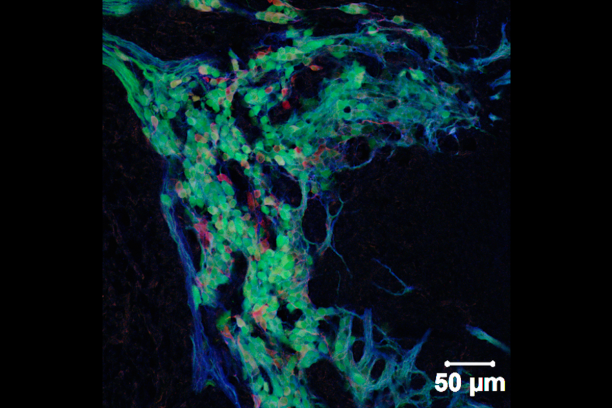
Innervation of visceral organs, like that in the intestine and bladder, is essential for normal digestion and elimination of waste products from the body. The neurons and glia that make up the peripheral ganglia in these organs derive from neural crest stem cells during fetal development. Our group uses developmental genetic approaches in the mouse to identify genes, gene interactions, and signaling pathways that impact the development of neural crest progenitors as they undergo migration and differentiation within these organ systems.
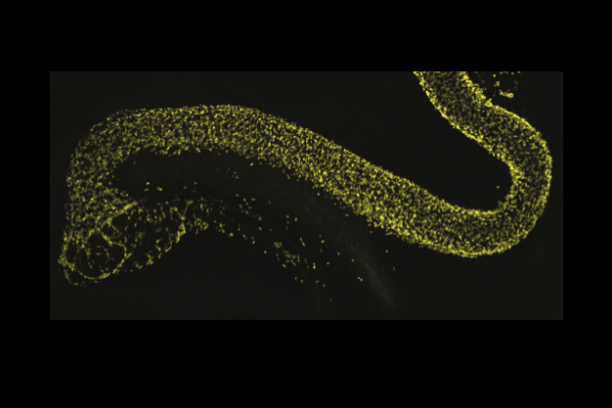
Sox10 is a transcription factor that is expressed in neural crest stem cells when they first form atop the neural tube in the developing embryo and is maintained as these progenitors migrate out to form elements of the peripheral nervous system. Sox10 is essential for normal formation of the enteric nervous system and its expression is maintained in mature enteric glia while it is turned off in enteric neurons. We have developed mouse transgenic and knock-in models that allow us to visualize cells expressing Sox10 and manipulate Sox10 expression levels. Our studies with these tools indicate that Sox10 not only affects the initial migration of neural crest stem cells but also impacts the balance of enteric neurons and glia that are present later in the postnatal intestine. Current efforts in the lab are aimed at using these tools and gene expression analyses in inbred strains of mice to better understand the processes that regulate normal neural crest cell differentiation as well as the mechanisms that cause gastrointestinal dysmotility.
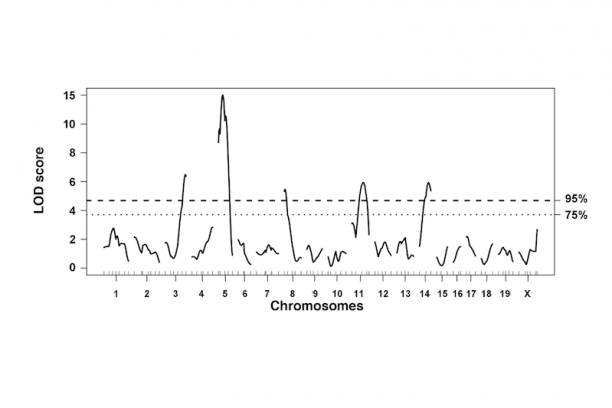
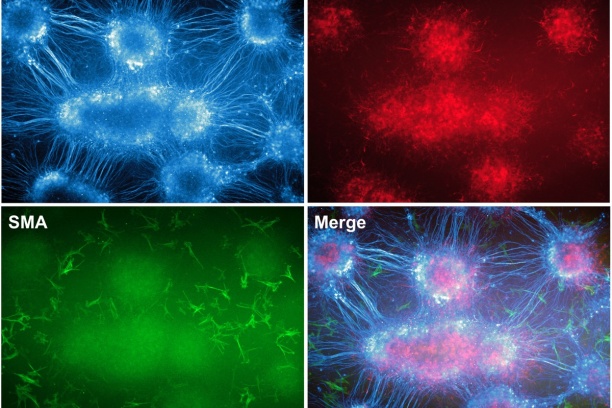
Our team is also focused on defining the cell types ("lineages") that come from neural crest in the bladder wall and urethra. Sox10-positive neural crest cells in the sacral region of the embryo also populate the lower urinary tract but until recently very little has been known about the timing, migration routes, signaling pathways, or genes that regulate differentiation of progenitors in this organ system. To better understand how developmental alterations of neural crest lead to lower urinary tract dysfunction. We are pursuing strategies to derive a comprehensive map of all the lower urinary tract cell types that derive from neural crest in normal development and in mouse models of Spina bifida. These studies are designed to determine the underlying mechanisms that predispose to altered bladder contractility in Spina bifida so that urologists may better treat neurogenic bladder that often occurs in these patients. As a means to identify the signaling pathways that regulate the migration and differentiation of sacral neural crest into the bladder we are capturing these progenitors by flow sorting and generating transcriptional profiles via RNASeq. In vitro pharmacological studies using explants of pelvic ganglia are being used to study specific effects of sacral neural crest signaling pathways on migration and differentiation.