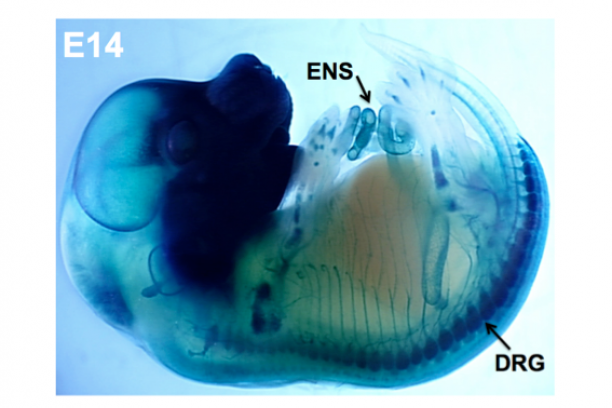
Our group is focused on understanding how discrete disease genes work individually and in combination with other genes in the genetic background to produce deficits of the peripheral nervous system. Our efforts are concentrated on two aspects of visceral organ innervation: the enteric nervous system (ENS), a network of interconnected ganglia in the wall of the intestine that controls gut motility; and pelvic nerves that control bladder emptying in the lower urinary tract (LUT). Peripheral ganglia in both of these systems derive from neural crest progenitors that migrate into these organs during development. We apply genetic and embryologic approaches using mouse models to define normal processes of neural crest differentiation and determine the pathophysiological effects of specific mutations on function of the mature intestine and bladder.
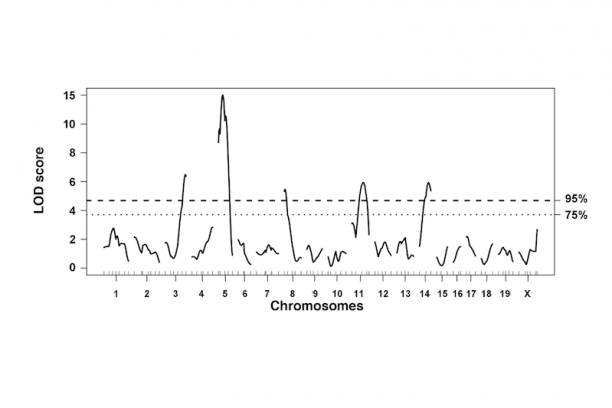
In the intestine, the ENS controls many essential functions including peristalsis, mucosal transport, tissue defense and vascular perfusion of the gut wall. Deficits in enteric neural crest development can produce ENS phenotypes that range from the extreme seen in Hirschsprung disease where enteric ganglia are completely lacking from the distal end of the intestine to abnormalities of ganglia size and distribution that are seen in idiopathic childhood chronic constipation. Even in families segregating a single Hirschsprung mutation there can be considerable variability between penetrance and extent of gut length affected by absence of enteric ganglia. To better understand the gene interactions that lead to such variability in patient phenotypes we are studying mouse models of Hirschsprung disease. We combine genetic strategies such as multiple cross mapping and outbred crosses with analysis of differentially expressed genes to identify genetic variants that affect neural crest development and increase susceptibility to aganglionosis. Concurrently we are investigating lineage segregation of neural crest in Hirschsprung mouse models to understand why so many of these patients continue to suffer from intestinal dysmotility long after surgical resection of distal aganglionic regions.
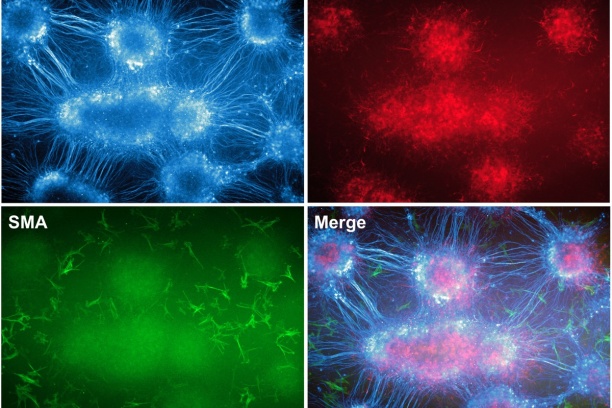
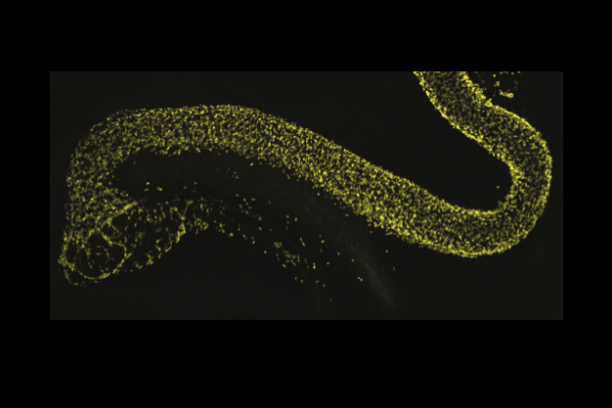
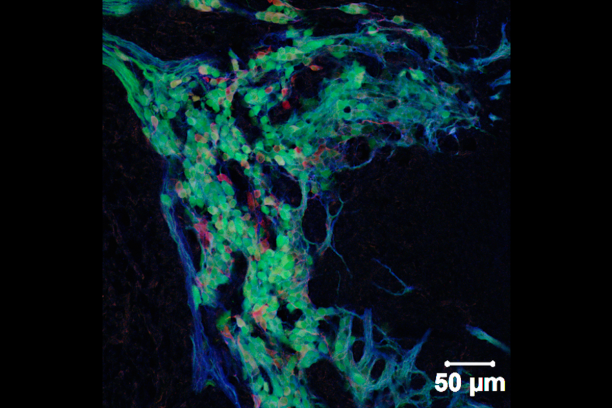
Appropriate innervation of the LUT is essential for bladder function and normal urination. We are working to define developmental mechanisms that regulate LUT innervation so that we can better understand how developmental alterations in this system lead to dysfunction. Initially we are pursuing lineage tracing strategies to derive a comprehensive temporal fate map of neural crest derivatives in normal LUT development and in mouse models of Spina bifida. Through these studies we have determined that neural crest progenitors in Spina bifida mutants are delayed in their migration into the bladder wall and undergo inappropriate differentiation. We are identifying the signaling pathways that regulate the migration and differentiation of LUT neural progenitors by cell sorting to achieve transcriptional profiling and in vitro pharamacological studies. Our analysis has identified several pathways that previously were not known to regulate peripheral neurogenesis in the LUT and should aid urologists in treating bladder dysfunction.