Below is an extensive list of research-related resources for researchers at Vanderbilt University Medical Center (VUMC). Most are specific to VUMC/Vanderbilt or Meharry and only available to members of those institutions. Many require login with an active VUNetID/VUMC ID to view.
For resources specific to the Vanderbilt Orthopaedics Department, please visit:
Vanderbilt Orthopaedics Clinical Research (OCR)
-
DISCOVR-e (IRB Submission Portal)
The Vanderbilt University Medical Center IRB's web portal for submissions, amendments, continuing reviews, and study closures.
- DISCOVR-e Help – User manual, FAQs, and info regarding department chair/division chief sign-off
eConsent
Vanderbilt has developed an eConsent framework within the REDCap platform, allowing research participants to rapidly review and sign consent documentation via web, tablet or smartphone. Electronic consent forms can leverage customized hover and click/pop outs, multi-lingual language capacity; text-to-speech accessibility options, “wet” signatures, and more, It can also be configured to be FDA Part 11 compliant.
- VICTR Guide to Using REDCap for eConsent – Comprehensive overview of setting up eConsents, submitting them to the IRB, using them.
- REDCap eConsent Data Dictionaries - Scroll to the bottom of the survey to download data dictionaries, then customize them for your project vs starting from scratch.
IMPORTANT NOTE: The data dictionary for the 1/21/19 version of the VUMC Health Sciences Consent Template does not include all currently required VUMC consent template language. Please see the VUMC HRPP website for the most up-to-date template version. - REDCap eConsent & Part 11 Compliance Guide – Guide to using eConsent in compliance with FDA Title CRF 21 Part 11, which governs the use of electronic records and signatures for FDA-regulated studies (e.g., all drug and device studies)
- Shareable eConsent Part-11 Documents – Scroll to the bottom of the form to download documents.
- eConsent Versioning Guide – Guide to maintaining versions in digital consent forms.
- Contactless Consenting Guide – Guide to using eConsent for contactless consent processes (i.e., subject and person obtaining consent are not in the same location).
- eConsent Workflow Considerations – List of considerations when thinking about using eConsent for your study.
Human Research Protections Program (HRPP)
Vanderbilt's Institutional Review Board (IRB). The IRB, also known as the independent ethics committee, ethical review board or research ethics board is an administrative body that applies research ethics by reviewing the methods proposed for research projects. The IRB protects the rights and welfare of human subjects participating in research activities. The IRB has the authority to approve, require modifications (to secure approval) or disapprove research.
- Applications and Consents – Consent form templates and IRB forms (HIPAA Authorization, genetic rider, etc.)
- Template Language and General Guidance – Template language for consent and IRB applications
- Tool Kit – Protocol development tools/templates, investigator’s handbook, guide to determining the appropriate IRB application for your research, info & resources
- Glossary of Terms – Glossary of IRB-related terms and acronyms
- Examples – Examples of consent/assent forms, adverse event (AE) Logs, and various scripts that can be modified to fit your study
- Text Readability Checker – Online tool for determining the readability level of patient-facing written materials (in general, investigators should aim for a 6th-8th grade reading level)
IRB Exchange (IREx)
Web-based portal supporting single IRB review documentation and coordination for multi-center clinical trials. IREx was launched in February 2017 by VUMC as part of the Trial Innovation Network. The IREx supports the use of the SMART IRB Agreement. IREx is also developing new functionality to allow for the tracking of non-SMART IRB Agreements (e.g., one-off MOUs) for sites that are unable to sign onto the SMART IRB Agreement.
- IREx FAQs – Frequently Asked Questions about setting up and using IREx for your multisite, single IRB study.
- Lead Study Team/Coordinating Resources – Info and resources for the lead site / coordinating center study team of a multisite study with a single IRB.
- Participating Site Study Team Resources – Info and resources for relying sites participating in a multisite study with a single IRB.
- IREx Training – Watch training videos, sign up for a live, monthly training sessions (based on role), or request a live IREx demo for your team.
NIH Certificate of Confidentiality (CoC)
Effective October 1, 2017, certificates of confidentiality are automatically issued by the NIH for all NIH-funded research involving human subjects, use of data at the individual level or biospecimens that are potentially identifiable, etc. A certificate of confidentiality places specific restrictions on the disclosure of identifiable, sensitive information that both researchers and their sub awardees must abide by.
Single IRB (sIRB)
As of January 25, 2018, NIH policy requires that a single IRB (sIRB) be used for all NIH-funded multisite research. The Vanderbilt IRB currently will only serve as the sIRB or cede reliance to another sIRB for federally-funded studies.
- Single IRB (sIRB) Help – Information and resources for study teams using a single IRB for multi-site studies either as a lead site or a relying site.
- sIRB Submission Tip Sheet for Lead Study Team when Vanderbilt is the sIRB
- sIRB Tip Sheet when Vanderbilt is Relying on Another IRB
- sIRB Letter of Support – for grant submissions (when Vanderbilt will be the sIRB).
SMART IRB (National Single IRB Initiative)
Single IRB reliance platform used by VUMC’s IRB for multisite research conducted under a single IRB. Participating institutions – both the lead site and relying sites – must sign the SMART IRB Agreement. Vanderbilt also requires a signed Letter of Indemnification (LOI) from all relying sites when Vanderbilt serves as the sIRB.
-
BioVU
Vanderbilt's biobank of de-identified DNA extracted from leftover and otherwise discarded clinical blood specimens. BioVU operates as a consented biorepository; all individuals must sign the BioVU consent form here in order to donate future specimens. BioVU subjects are de-identified and linked to the Synthetic Derivative (SD) which enables researchers to access genetic data/DNA material and longitudinal electronic medical record (EMR) information. Access to BioVU requires appropriate IRB approval as well as an application (which undergoes administrative & scientific review).
- BioVU Access Application – Overview and instructions for obtaining BioVu access
Clinical Data Interoperability Services (CDIS) - Clinical Data Pull & Clinical Data Mart
The Clinical Data Pull and Clinical Data Mart from eStar are special features that import clinical data from Epic (eStar) into REDCap. Both features fall under the umbrella of Clinical Data Interoperability Services. CDIS is an optional feature, most useful for REDCap projects requiring frequent updating of clinical data in many REDCap fields/records. Datapoints that you can pull into REDCap from Epic include: (1) Demographics, (2) Current Problems list, (3) Medications list, (4) Allergy Intolerance list, (5) Vital Signs, (6) Laboratory, (7) Adverse Event, (8) Core Characteristics (e.g., birth weight), (9) Encounter List, and (10) Immunizations. For more information, see the CDIS section of the Vanderbilt REDCap User Guide. Use of CDIS is free but requires appropriate IRB approval.
- Clinical Data Pull (CDP) - Clinical Data Pull is a special feature for importing data into an existing REDCap project from an EHR (electronic health record system), such as Epic, Cerner, etc. It provides an adjudication process whereby REDCap users can approve all incoming data from the EHR before it is officially saved in their REDCap project. Clinical Data Pull can only be enabled by a REDCap Administrator, so you should contact them if you wish to utilize Clinical Data Pull for your project.
- Clinical Data Mart (CDM) - CDM is a special feature that imports eStar clinical data into a totally new REDCap project at the time the project is first created. The project creation includes setting up a 'fetch' request, which includes things like the list of Medical Record Numbers in your target dataset, data types and values, and the date range. CDM is most useful for retrospective research studies, where you want to pull an eStar dataset once and then rarely (or never) update it by pulling those values again later. CDM differs from CDP in that the REDCap project is created AFTER the CDM feature is enabled on your user account. So you should submit your CDM request BEFORE creating your REDCap project.
- Monthly Live REDCap/eSTAR Integration & Use Workshop – Monthly workshop that consists of an interactive demo and advanced view into the functions of CDIS. Topics covered include: (1) Steps for CDIS Activation; (2) How to set up your REDCap project; and (3) How to add patients to the project. Registration required.
Crowdsourcing
Crowdsourcing is a billable service that engages medical students and graduate nursing students to review medical documents. Crowdsourcing is well suited to projects that require manual review of: (1) Medical record documents (such as pathology reports), (2) Imaging studies, (3) Notes in the medical chart, or (4) Other pieces of the medical chart. For identified data, reviewers must be added as KSP to a study’s IRB and must sign a DUA.
External Research Data Sharing
Information regarding sharing any research data external to Vanderbilt, including VUMC External Data Sharing Policy and external data sharing tool designed to guide researchers through VUMC’s external data sharing expectations. Any research data leaving VUMC – including fully de-identified data – is expected to be associated with an appropriate legal agreement (e.g., Research Contract, Clinical Trial Agreement (CTA), Data Use Agreement (DUA), Material Transfer Agreement (MTA)) and should be consistent with the language in the informed consent. In general, unless there is a consent specifically allowing sharing of data, only de-identified data may be shared. Aggregate data can be shared without a written agreement, but any data at the individual level (even if fully de-identified) requires a formal agreement.
- VUMC External Data Sharing Tool – 3-minute survey that will yield an external data sharing plan for your study
- Clinical Trial Agreements (CTA)
- Data Use Agreements (DUA)
- Material Transfer Agreements (MTA)
- Human Data Risk Assessment Tool (HDRAT) - instructions for completing the HDRAT in a contract or DUA request in PEER
ImageVU
ImageVU is a medical imaging repository linked to identified clinical data through the Research Derivative (RD). ImageVU consists of magnetic resonance imaging (MRI) and computerized tomography (CT), along with image metadata including study description, exam codes, and image acquisition date and time. Currently, ImageVU contains over 7.4 million MRI and CT image series, from 826,000 studies performed on 246 patients, dating back to 2007.
MyCap
MyCap is an electronic platform designed to let researchers capture patient reported outcomes and data via a mobile app installed on participants' devices (Android or iOS). Researchers use the MyCap external module for REDCap to configure how their project should look and behave within the MyCap mobile app. Participants download the mobile app and complete tasks/surveys. Task results are synchronized to the researcher's REDCap project.
- MyCap Public Webpage – Info & demos
- MyCap Resources – Overview materials, User Guide, Quick Guides, and Decision Tree (to determine whether MyCap is well-suited to your project)
- MyCap FAQs
Record Counter
Part of the Synthetic Derivative (SD). Record Counter provides exploratory data figures to members of the Vanderbilt research community for research planning purposes and feasibility assessment. It allows the user to input basic medical data such as ICD 9 codes or text keywords (e.g., lung cancer) as well and demographic info and then search the SD database to determine the approximate number of records that meet those criteria. Anyone with VUnetID/VUMC ID can use Record Counter. Use of Record Counter is free and does not require IRB approval.
REDCap (Research Electronic Data Capture)
REDCap is a secure web platform for building and managing online databases and surveys. REDCap's streamlined process for rapidly creating and designing projects offers a vast array of tools that can be tailored to virtually any data collection strategy. All Vanderbilt faculty, staff, students, trainees & affiliates have REDCap access. REDCap databases can also be configured to pull data directly from the electronic medical record (Epic). Please note that any publication that results from a project utilizing REDCap should cite Grant support (UL1 TR000445 from NCATS/NIH).
- Training Resources – Library of REDCap training videos. Accessible directly within REDCap by clicking “Video Tutorials” in the “Help & Information” section of the main menu panel.
- Additional Training Videos – Video recordings of live training sessions conducted for VUMC users are located in the Vanderbilt/Meharry User FAQ Pages, under Help Resources.
- REDCap Help & FAQs (login to REDCap required to access, then, click "Help & FAQ's" at top) – Searchable database providing detailed overview/instructions for most REDCap features. Accessible directly within REDCap by clicking “Help & FAQ” from the “Help & Information” section of the main menu panel.
- Vanderbilt/Meharry User FAQs – Detailed Frequently Asked Questions specific to Vanderbilt/Meharry users and accounts. Provides detailed info about features, functions, and resources specific to the Vanderbilt installation of REDCap. Housed within ROCKET.
- REDCap User Guide (for VUMC) – Comprehensive, downloadable user guide for Vanderbilt users.
- REDCap Day is held annually in February at VUMC and is a great way to learn about upcoming features in REDCap, interesting use cases, and attend breakout sessions on REDCap topics of interest.
Research Derivative (RD)
Vanderbilt’s fully identified repository of clinical data drawn from the electronic medical record (Epic, StarPanel, the Enterprise Data Warehouse (EDW), HEO, and other sources). RD data is updated regularly and typically includes data from about 4 weeks prior to the update date. The RD is suited for projects requiring identified information for linkage to other data sources, large cohort extraction, and other projects requiring specialized data sets. The RD can be used for: (1) Identification of large subject cohorts and extraction of data sets; (2) supplement data for studies with a pre-defined cohort that requires additional variables; or (3) recruitment for clinical studies by combining clinical and appointment data. Use of the RD is free but requires appropriate IRB approval.
Synthetic Derivative (SD)
Vanderbilt’s fully de-identified repository of clinical data. The SD is a multi-source repository of fully de-identified clinical data from across the Vanderbilt enterprise. The database contains over 3.2 million electronic medical records (approximately 1 million+ with detailed longitudinal data), and data is updated bimonthly. The SD also contains integrated genetic data made available through BioVU. The SD can be used for: (1) cohort identification and data extraction for non-human study research, and (2) connecting patient data to genomic data in BioVU to study the links between genes and disease/medicine. Use of the SD is free, but requires appropriate IRB approval.
Vanderbilt Clinical Informatics Center (VCLIC)
VCLIC is a clinical informatic core, based in the Department of Biomedical Informatics. The mission of VCLIC’s Clinical Informatics Core is to enable VUMC researchers to design and implement electronic health record (EHR)-related tools, functionalities, and interventions with input and assistance from clinical informatics experts, as well as to gain access to and analyze EHR-based data. The core offers: (1) Clinical data extraction and analysis, (2) Design and build of EHR interventions, (3) Predictive model implementation, (4) Value set and logic development, and (5) General clinical informatics consultation. Services are available for a fee, and, in many cases can be funded through a VICTR voucher.
Vanderbilt Department of Biostatistics
The Vanderbilt Department of Biostatistics offers several tools and services to the Vanderbilt research community.
- Power & Sample Size Tool (PS) – An interactive program for performing power and sample size calculations. It can be run off the internet or downloaded for free.
- Biostatistics Clinics (M-F noon-1:15pm) – A free service staffed by the Dept of Biostatistics open to all members of the Vanderbilt/Meharry communities who have methodologic questions about their research projects or about published articles. Mentors are required to attend clinics with all trainees (e.g., students, residents, postdoc fellows). Registration is required. Note: Best day for Orthopaedic studies is Thursday.
- Biostats Project Support/Consultation Funded thru VICTR Resource Requests (VRR) – Guidelines for VICTR biostats support application for up to $5,000 (90 hrs) VICTR support to provide biostatistical consulting/support by a staff biostatistician.
- Statistical Computing Series – A monthly event for learning various aspects of modern statistical computing from practitioners in the Department of Biostatistics focused on topics related to the R language, Python, and related tools.
-
Edge for Scholars
Grant writing resources and services free to VUMC faculty, fellows and others.
- Funded Grants Library – A repository of successfully funded R, K, and other awards, including some non-NIH available for review
- Edge Reviews – NIH-like internal study sections by senior Vanderbilt faculty are conducted six times a year in advance of NIH cycles for first-submission R and K awards, resubmissions, revisions, and renewals for basic and clinical investigation
- Grant Pacing Workshops – A practical series of workshops and peer accountability meetings designed to pace attendees smoothly to the completion of a grant. Topics covered include making a customized
Tools for Grant Writing
List of resources and services compiled by VICTR, including available pre-submission resources and services, grant-ready text, and other grant resources from a variety of VUMC sources.
VICTR Funding Program
Vanderbilt Institute for Clinical and Translational Research (VICTR) fundable studies are clinical and translational hypothesis driven projects that involve human subjects, human tissue, human cell lines, human information (e.g., medical records), and have application to human health. Providing access to funds to generate pilot and preliminary data is a vital component of the VICTR mission. All VICTR applications should be limited to pilot-level, with the exception of sponsored requests for access to the Clinical Research Center (CRC).
- VICTR Funding (VICTR Resource Requests) – VICTR funding eligibility and review criteria, allowable resources, and application process for VICTR Resource Requests (VRR)
- VICTR Funding Guidelines – VICTR funding eligibility and resources available/not available via VICTR funding, review
- VICTR Funding Resource Request Instructions – PowerPoint presentation providing step-by-step instructions for completing and submitting a VICTR Resource Request (VRR)
- VICTR Resource Request (VRR) – Submission Portal - where to go when you are ready to apply for VICTR funding
- VICTR Funding Templates, Forms & Examples – Multiple resources including “Before You Apply Checklist”, Research Proposal template & sample, and much more
- VICTR Funding Overview Training Sessions – Monthly live clinic to learn and ask questions about the VICTR Funding Program (no registration required)
-
Clinical Research Fiscal Support Services (CRFSS)
A centralized group available to assist with clinical trial billing and budgeting needs, such as eSMART submissions, budget development and/or negotiation, billing grid creation, investigational device set up, use of eSTAR for research, customized education sessions, etc. CRFSS also provides daily office hours via phone, Zoom or in person.
- CRFSS Resources – eSTAR & eSMART guides & tip sheets, telemedicine/teleconferencing guidance, archived presentations, training, eSMART/eSTAR updates and more
- CRFSS FAQs – Frequently Asked Questions for eSMART & eSTAR
- CRFSS Flyer – Overview of CRFSS services offered
Clinical Trials Billing Compliance
The Clinical Trials Billing Compliance (CTBC) team works to ensure that charges are routed correctly and patients or funding sources are not inappropriately charged for patient care services. For all interventional studies (whether billable or not) and for all billable non-interventional studies, enrollment/consent statuses for participants must be maintained within eSTAR. Billable studies must also have a designated charge reconciliation owner who regularly performs, clinical trial charge reconciliation (See “Clinical Trials Charge Reconciliation”).
- Enrollment Status Maintenance - Detailed information and training regarding maintaining enrollment/consent for research participants in eSTAR
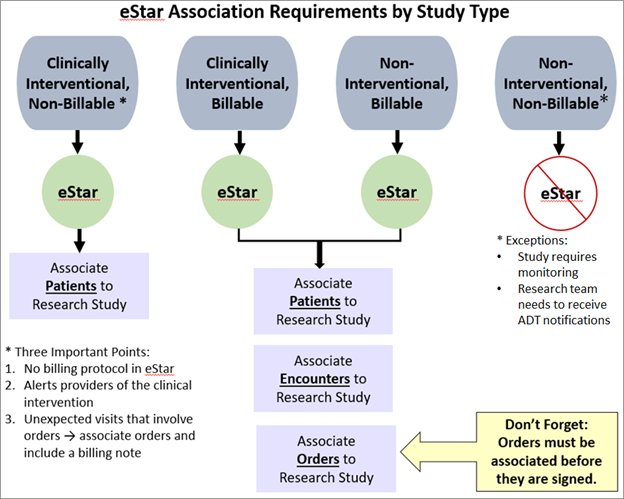
Clinical Trials Change Reconciliation
VUMC policy requires weekly Clinical Trials Charge Reconciliation of all patient care charges on a research study to ensure the charges were routed properly. The purpose of reviewing the charges weekly is to ensure billing errors are identified and corrected quickly, especially those that route to the research center in error and should have routed to insurance (or vice versa) and ensure charges routed incorrectly are corrected in a timely, compliant manner. It is illegal to bill Medicare/Medicaid or any other third-party payers for expenses that are intended for clinical study sponsors.
All billable studies must have a designated charge reconciliation owner, and clinical trial charge reconciliation must be performed weekly. A REDCap survey is sent to all charge reconciliation owners every two weeks and must be completed to attest that weekly charge reconciliation has been performed for the survey period.
- Charge Reconciliation Policies & Resources – comprehensive overview and links to various resources
- Charge Reconciliation Quick Guides & Training
- Charge Reconciliation Owner Attestation Guide
- Charge Reconciliation Reports Guide
COEUS
COEUS is Vanderbilt’s online grant submission application.
eSMART
A VUMC electronic research management system required for the initiation of ALL human subjects research that optimizes compliance between various regulatory processes necessary for conducting clinical research. eSMART shares data with multiple system, including eSTAR, PEER, DISCOVR-e, COEUS and VICTR and enables researchers to track their clinical research studies online. It provides data for eStar study set-up, including the routing of research related patient care charges.
- Intro to eSMART Presentation- PowerPoint presentation providing guidance on how to navigate the eSMART system
- eSMART User Guide
- eSMART (Login)
eSMART Invoicing Module
Newly released invoicing module within eSMART to help study teams compliantly manage.clinical trial sponsor invoicing. The module provides a uniform template that includes both VUMC-specific and funding source-required info, sequentially numbers invoices, and supports tracking and reporting for invoiced and automatic milestone payments documenting receipt of payments. Registration for eSMART invoicing training workshops is available via Learning Exchange.
- Clinical Trial Financial Management Expectations – reminders from CRFSS re: invoicing and fixed price payments
Office of Grants and Contract Management (OCM)
A division of the VUMC Office of Research. Serves as the central Medical Center office for contract intake, processing, negotiation and management. OCM reviews both research and non-research contracts. Research agreements include bench research, clinical trials, federal contracts and federal subcontracts. Non-research agreements include professional services, educational and affiliation agreements.
- FAQs – Frequently Asked Questions about contract review & processing
- VUMC Payment Addresses – for invoices and payments by ACH, US Mail, or Overnight/Express
- Research Agreements – Information about (1) Clinical Trial Agreements, (2) Federal Subcontracts, and (3) Industry (Non-clinical)
- Other Agreements – Information about other agreements such as Confidentiality agreements, Data Use Agreements, etc.
Office for Sponsored Programs (OSP)
The Office of Sponsored Programs (OSP) assists all members of the VUMC community with the submission of grant proposals for external support of research, creative, instruction, and service activities. OSP supports investigators who have or plan to apply for external funding and departmental administrators who assist these investigators with proposal preparation and award administration.
- Forms – Most recent versions of (1) VUMC Fact Sheet (Payment Info, Identifiers & Contacts), (2) Current VUMC Rate Agreement (including IDC and fringe rates), (3) internal budget forms, (4) Just-in-Time (JIT) resources, and (5) Letter of Intent (LOI) resources
- Training & Checklists – Education info as well as checklists for the following: (1) Biosketches, (2) Equipment Requests, (3) Foreign Travel Requests, (4) No-Cost Extension Requests, (5) Pre-Award Spending, and (6) Other Support Templates
- Grant Cycle – Info about the Grant cycle from finding funding and preparing a proposal to setting up, managing, and closing out a grant award
- Policies and Guidance – Current F&A rate agreements and fringe benefit rates, uniform guidance audit, and various policies
- Coeus Help – Coeus grant system resources, forms, reports, training, FAQs, and contract info
PEER
PEER (Paperless Environment for Electronic Review) is a web portal developed and maintained by the VUMC Office of Contract Management to streamline submission and management of grant-and contract-related requests and information. The information collected via PEER include Conflict of Interest Certifications and Investigator Assurances for grant applications, new contract requests, and other award management items such as grant re-budget requests, effort changes, and more.
Price Checker
Online tool for access to research-discounted prices for procedures, professional services, medications, etc. used in the conduct of research studies at Vanderbilt. A useful tool when creating research study budgets. Custom pricing requests can be submitted for items not already listed via My Pricing Requests.
-
EHR-based Recruitment Tools (via eSTAR & MHAV)
The EHR Recruitment Support team can provide tools for leveraging our electronic health record (EHR) via eSTAR and MyHealth at Vanderbilt (MHAV) to support research participant recruitment. EHR tools can be use to identify, scree, and/or recruit participants.- eSTAR Reporting Workbench – Customized reports in eSTAR based on study inclusion/exclusion criteria that can also include variables such as Research OK to Contact and MHAV messaging status to help study team determine appropriate outreach methods
- MyHealth at Vanderbilt Mass Messaging – Tool for sending research recruitment messages to a predefined cohort of patients who (1) meet study inclusion/exclusion criteria, (2) have given explicit OK to contact for research, and (3) have an active MHAV account
- MyHealth at Vanderbilt Individual Direct Patient Messaging – Tool for messaging specific patients directly through their MHAV account. Primarily used for patients with whom members of the study team have an existing healthcare provider relationship
- EHR/MHAV-based Recruitment Resources (Survey) – Intake form to help determine which electronic health record-based recruitment tool would best support your study’s specific needs.
eSTAR (accessed via virtual desktop or browser)
eSTAR is Vanderbilt University Medical Center’s Epic-based electronic health record (EHR) system. With IRB approval, clinic schedules and patient records can be reviewed by authorized research personnel to identify potential research study participants. There are two basic levels of research access: (1) Chart Review Only (read only access), which requires only online training; and (2) Coordinator access (read-write access), which requires both online and scheduled live training.
- Hubbl – eStar Tip Sheets that detail specific functionality within eStar are available via Hubbl. To locate a Tip Sheet, log in to Hubble and select the Tip Sheets hyperlink. In the Search for tipsheets bar, enter a search term or eStar application name to be presented with the existing Tip Sheets. Once a Tip Sheet has been located, it can be saved for future reference.
- eSTAR Research Training & Security Access – REDCap form to request eSTAR research access or to register for eSTAR Research training.
- eStar IT Support for Providers – Overview of eStar resources – including 1:1 Training Sessions - available for providers/clinicians.
Research Match
ResearchMatch is a free and secure online tool that helps match willing volunteers with eligible researchers and their studies at institutions across the country (Over 8,000 volunteers live within 50 miles of VUMC). ResearchMatch provides researchers at participating institutions a free tool for participant recruitment and feasibility analysis.
Research Notifications Distribution List
The Research Notification Distribution List is a free recruitment tool for Vanderbilt researchers to contact over 18,000 participants (Vanderbilt/VUMC faculty and staff and members of the community in Middle Tennessee). Research teams may request to have their IRB-approved participant recruitment flyer/email sent via this distribution tool. Studies must have IRB approval to use the Research Notifications Distribution list and must be registered on ResearchMatch.
Research Participant Compensation
Comprehensive resources and guidelines related to participant compensation and the various methods that can be used for this purpose.
Social Media Toolkit
VUMC social media policy information and an application to use Facebook ads for your research study. Facebook ad campaigns for research are managed by VUMC’s Strategic Marketing Services but paid for by the study.
Subject Locator
A tool for identifying potential study subjects at Vanderbilt outpatient clinics. Based on a list of upcoming appointments in a predetermined set of clinics, Subject Locator searches patients’ Epic records for commonly used, discrete inclusion/exclusion criteria to significantly narrow down the number of patients that require screening. Subject locator is available to those with all of the following: (1) a Vanderbilt IRB-approved research study, (2) IRB approval to use Subject Locator in recruiting for the study, (3) Access to Epic, and (4) a valid VUNet/VUMC ID.
Trial Innovation Network (TIN) Toolbox
Free resource to the research community that collects and compiles recruitment and retention materials and strategies from CTSAs and reputable community health partners. It includes guidance and templates for everything from developing a study website, using digital tools for recruitment & consent, recruiting minority and pediatric populations, reaching out to referring providers, sharing study results, and more.
Vanderbilt Clinical Trials Directory
VUMC website displaying all actively recruiting Vanderbilt clinical trials. Only displays trials that are registered in ClinicalTrials.gov and whose status is actively recruiting.
-
iLab
Online portal to request VUMC Cores services and track billing.
Medical Products Support Services (MPSS)
Medical Products Support Services (located within the Vanderbilt Center for Technology Transfer and Commercialization) provides free assistant to Vanderbilt investigators in two areas: (1) the Medical Device Regulatory Affairs Program (MDRAP), and (2) Medical Products Development and Commercialization Program (MPDCP). The former provides help regarding medical device regulation, including discussion of regulatory strategies and device classification, assistance with application submissions, and communication with the Vanderbilt IRB and the FDA. The latter assists with all types of medical product development, offering help with regulatory, manufacturing, and commercialization steps as well as providing a statement of support for grant purposes, if needed.
Research Support Services (RSS)
The mission of the Research Support Services office is to assist investigators in navigating the complex human research process at Vanderbilt. Research Services consultants experienced in conducting research or IRB activities provide guidance in the following areas: (1) Study organization, (2) IRB navigation, (3) Training, (4) Protocol development, (5) Preparation of study-related documents, (6) Funding and contractual agreements, and (7) Overall program advisory (IMPACTT). Research Services Consultants (RSC) are available to provide individualized consultation by telephone, email, or in person on a variety of research-related topics.
Scientific Poster Printing
Scientific poster printing services are available via the on-campus sources below. Additional poster resources, including templates and tips are available to Ortho research personnel on this site’s OCR Research Resources page.
- Biomedical Research Education & Training (BRET) Office Poster Printing - Non-profit poster printing service for affiliates of the biomedical sciences graduate programs at Vanderbilt offering low-cost, fast turnaround printing of scientific posters for presentations and conferences
- VICTR Scientific Poster Printing – Clinical Research Center (CRC) poster printing services are available to researchers who were awarded VICTR Resources
StarBrite
StarBRITE is a one-stop, web-based research portal designed to assist the Vanderbilt University and Meharry Medical College research community during the planning and conduct of research studies. StarBRITE assists with identification and location of resources, identification of experts, guidance for regulatory applications and approvals, regulatory assistance, funding requests, research data planning and collection, and serves as a central repository for educational offerings VICTR info and resources are available via StarBRITE as well as tip, templates, and toolkits for every stage of study development and execution.
- Research Project Planning & Implementation Resources – a myriad of resources for every stage in the research project development cycle, including tools for: (1) Idea / Feasibility, (2) Development, (3) Implementation, (4) Analysis / Close Out, (5) Dissemination.
- Study Document Templates – Templates for (1) grant submissions components (e.g., recruitment & retention plan, clinical & data monitoring plan, protection of human subjects, etc.); (2) pre-study activities (such as Confidentiality & materials transfer agreements); (3) study activities (e.g., consent forms, protocol, case report forms, delegation of authority log, regulatory binder checklist/compliance worksheet, etc.), and (4) post-study activities (such as study close out application)
- Recruitment Resources – List and links for various VUMC recruitment resources
- Funding Resources – Funding available through VICTR
- Data Management Resources – Overview of data collection and storage resources available at no cost to VUMC & Meharry research teams, including REDCap, MyCap, eConsent, the Research Derivative
- Education Resources – VUMC Research Education Calendar and resources
- VICTR Data Resources – Overview of VUMC data warehouse architecture and resources available to researchers for accessing both identified and de-identified data from the VUMC clinical enterprise
Studios
VICTR-sponsored studios are structured, dynamic sessions tailored for research investigators that bring together relevant research experts in a particular methodology to focus on a specific stage of research. Studios are intended to provide tools/feedback for investigators to use to enhance research quality, improve funding success, foster advances in clinical practice and improvements in patient health, increase publications and generate new hypotheses. Studio types include: (1) Community Engagement Studio, (2) Research Design Studio, (3) Grant Review Studio, (4) Hypothesis Generation Studio, (5) Implementation Studio, (6) Manuscript Studio, and (7) Specific Aims Studio. Participation in studios is funded through VICTR Resource Requests (VRR).
Vanderbilt Clinical Research Center (CRC)
The Vanderbilt Clinical Research Center is an inpatient and outpatient research facility staffed with personnel dedicated to conducting clinical research patient care. These resources are available to Vanderbilt University and Meharry Medical College clinical investigators and their study teams. Resources include exam rooms (including larger rooms suitable for infusions), a specimen processing lab, and meeting rooms appropriate for obtaining patient consent, consultations, patient interviews, and survey-based research studies. Services are provided at a subsidized rate for investigator-initiated and federally-funded research studies via VICTR Resource Request (VRR).
Vanderbilt Coordinating Center (VCC)
The Vanderbilt Coordinating Center (VCC) aims to provide flexible, comprehensive support for Vanderbilt investigators participating in clinical and translational research in their efforts to advance medical therapies and improve patient care around the globe. VCC services can be used to support basic, sample collection studies to the most complex study design and operational planning accommodating everything in-between. In addition to serving as a “one-stop-shop” for clinical and translational research needs, the VCC also provides flexible project support with the option to “Plug and Play” select services as needed. Investigators requiring focused support for specific needs as well as those requesting full project support can all be executed by the VCC. The VCC is structured as a Federal Charge Back Core with federally compliant and revenue neutral rates.
- Local Support – Assistance to both new and experienced investigators with review, preparation, and conduct of clinical trials. Experienced, certified clinical research coordinators are available to provide complete study coordination, including: study feasibility, logistical considerations, IRB preparation and submission, study start-up, regulatory compliance, participant recruitment, data management, budgetary review and negotiation as well as financial management.
- Program for Investigator Initiated Trials – The VCC offers investigator-initiated trial support to investigators engaging in expanding their studies to sites on national and international platforms
- VCC Services & Rates – Service rates & descriptions
- Request for VCC Services – Overview of request process & application survey
Vanderbilt Institute for Clinical and Translational Research (VICTR)
A virtual home for clinical and translational research at Vanderbilt. VICTR is funded by the National Center for Advancing Translational Sciences (NCATS) Clinical Translational Science Award (CTSA) Program. Its mission is to transform the way ideas and research discoveries make their way from origin to patient care. VICTR helps provide tools and support to improve the quality of research through collaboration with a wide variety of research partners, training participating researchers, funding research, developing new informatics and biostatistical systems, and more. VICTR info and resources are available via StarBRITE. The CTSA grant must be cited if any VICTR funding was received or any VICTR services were used to support the research project.
- Citing the CTSA Grant – Information and examples for proper citation of the VICTR CTSA grant.
Vanderbilt Technologies for Advanced Genomics (VANTAGE)
VANTAGE is a one-stop genomics resource, including: illumina genotyping, DNA/RNA extraction and banking, Affymetrix-based genotyping and expression microarrays, Sanger sequencing, and Next Generation Sequencing (NGS), among many other specialized services.
Vanderbilt University Institute of Imaging Science (VUIIS)
VUIIS operates state-of-the-art facilities for imaging research at all scales including imaging animals and human subjects. VUIIS pursues research in developing new imaging methods as well as applications in cancer, neuroscience, metabolic disorders, cardiovascular disease and other areas.
- Center for Human Imaging - The Human Imaging Core (HIC) provides resources for structural and functional imaging and spectroscopy. Magnetic resonance imaging (MRI) and spectroscopy (MRS) are available on 3, 7 Tesla full body scanners. HIC supports magnetic resonance imaging (MRI) and magnetic resonance spectroscopy (MRS) protocol development, functional MRI (fMRI) experimental design, subject preparation, structural and functional image analysis, and training on image analysis techniques and tools.
VUMC Research Cores
VUMC Research Cores and other shared resources offer cutting edge scientific services, enable access to high-end equipment, advanced techniques, and provide specialized expertise for all Vanderbilt investigators. Core services are set up and paid for through i-Lab.
-
Clinical Research Center (CRC) Research Skills Weekly Workshops
The weekly CRC Research Skills Workshops offer basic instruction and practical advice on commonly encountered clinical research topics. Sessions are typically demonstration-oriented and provide an informal setting to learn new skills. Sessions are free, open to anyone at VU, VUMC, or Meharry (there is no registration), and offer 1 CME credit per workshop. A list of upcoming workshops and instruction on how to sign up to receive weekly email reminders for workshop topics as well as archives of past workshop topics (with attached slides/handouts) and presentation recordings are available via the links below.
- Upcoming Workshop Topics
- Archived Workshop Topics with links to handouts
- Past Workshop Recordings – from Aug 2020 forward (in BOX)
- Past Workshop Recordings – prior to Aug 2020 (in ROCKET)
Clinical Research Immersion “Boot Camp”
This free, full-day session provides clinical research staff with the fundamentals of clinical research. It presents an overview of the requirements and processes necessary for the conduct of clinical research at VUMC, with direction to internal and external sources. All Clinical/Translational Research Coordinators are required to complete this training within their first year. Topics include: VUMC Research Resources, Responsibilities of the Study Team and Sponsor, Protocol Implementation, Study Documentation, Recruitment & Retention of Study Participants, Informed Consent Process and eConsent, and Adverse Event Reporting.
Human Subjects & Good Clinical Practice (GCP) Training (Required IRB training)
Vanderbilt University Medical Center IRB's website for human subjects, good clinical practice (GCP) and annual training information. Human subjects training is required for all human research studies and, as of 2021, must be renewed every 3 years (previously this training had to be renewed annually). GCP training is required for all clinical trials and must be renewed every three years.
Learning Exchange
VUMC’s primary online training portal, used to complete institutionally required compliance and service training as well as online training segments for eStar access. It provides online training and live training registration for a wide variety of VUMC processes, systems, and topics.
Vanderbilt Program in Research Administration Development (VPRAD)
- Intro to VPRAD is a four-hour training workshop what will acclimate enrolled staff to the VUMC Research Enterprise and introduce the resources available to research-related roles throughout VUMC. This course is intended for newly hired, recently transferred, or any other staff member working in an administrative role within a lab, clinic, or administrative office within the research enterprise. This workshop is offered at no cost to participants or their departments.
- VPRAD Level 1 is a professional development program at VUMC intended to fill in knowledge gaps and equip learners with a broad overview of VUMC’s research enterprise as well as the tools and resources to navigate within in. Participants spend 12 weeks learning topics including the lifecycle of a sponsored project, research compliance, shared resources, pre- and post-award management, financial processes, and VUMC best practices in research administration. VPRAD Level 1 is offered twice a year. Session are 4 hours per week, for 12 weeks and include classroom learning, a mentorship program, tours, and a final exam. Participation requires supervisor’s approval and a $150 fee.
VICTR Town Hall / RSS Presentations (Archived Presentations)
Archive of past Research Support Services (RSS) & VICTR Town Hall presentations including video recordings and slides.
VUMC Research Education & Training Resources Calendar
Enterprise-wide education calendar of research trainings for the Vanderbilt community.
-
ClinicalTrials.gov
Web-based resource provided by the US National Library of Medicine that provides the public with easy access to information on publicly and privately supported clinical studies on a wide range of diseases and conditions. All studies that meet the designated criteria must be registered in clinicaltrials.gov and results updated with the final study results. You may need to register your trial if any of the following apply: (1) You are investigating a drug, device, or biologic; (2) you will receive NIH funding (including VICTR funds) for the conduct of an interventional trial; or (3) you want to publish results (ICMJE).
- ClinicalTrials.gov VUMC Resources – FAQs, registration guide & templates for Vanderbilt researchers. Info on how to register & report results, penalties for not registering, etc.
- VUMC CT.gov Registration Survey – tool to help determine if your VUMC trial requires registration with Clinical Trials.gov.
- ClinicalTrials.gov website FAQs - Frequently Asked Questions on the official ClinicalTrials.gov website.
NIH Public Access Compliance
The NIH Public Access policy requires that the final, peer-reviewed journal article resulting from NIH-funded activities (this includes any VICTR funding) MUST be submitted to the PubMed Central (PMC) repository upon acceptance for publication and completed by at least 3 months post publication to remain compliant. The links below provide information, tools, and guidance regarding compliance with the NIH Public Access policy, including use of MyNCBI and PMCIDs.
- NIH Website Public Access Policy Resources – Overview, video training, and instructions on the NIH website for compliance with the NIH policy
- NIH Public Access Compliance Info – Information and tips for Vanderbilt researchers related to compliance with NIH public access policy.
- StarBRITE Publication Compliance Dashboard - A web-based tool for monitoring publication compliance in accordance with the NIH Public Access Policy
PubMed
Free full-text archive of biomedical and life sciences journal literature at the US National Institutes of Health’s National Library of Medicine.
Research Results Dissemination Resources & Toolkit
Information and resources for developing dissemination plans and products for research results, including strategies, examples, templates, and more compiled by VICTR.
Tools for Manuscript Writing
List of resources and services compiled by VICTR.
-
Cloud File Collaboration (OneDrive & SharePoint)
Cloud file collaboration allows team members to store, share, and edit files together at the same time using Microsoft cloud services OneDrive for Business (for your individual files) or SharePoint Online (for your department files). Microsoft Teams is a portal to content stored in OneDrive and SharePoint. The files themselves are not stored directly in Teams.
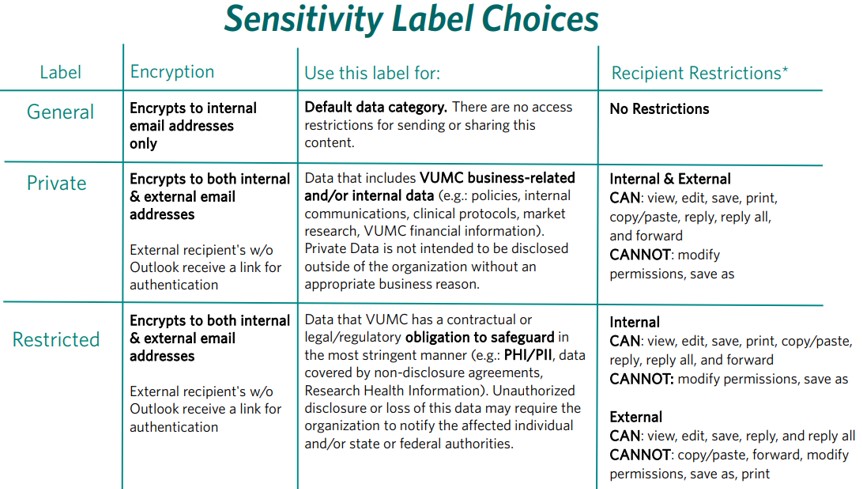
Outlook Sensitivity Settings
VUMC employees who want to send content (both email language & attachments) securely via email can do so using Sensitivity Labels within Microsoft Outlook by using the appropriate categories below. Email subject lines should not include any sensitive information such as PHI. The restricted label can be used as a secure replacement for Accelion.
Research Organization Collaboration and Knowledge Exchange Toolkit (ROCKET)
ROCKET is a Vanderbilt University Medical Center web-based tool for sharing information and documents, allowing members of a workspace to collaborate. It essentially works like an electronic bulletin board or website that can be public or shared only with specific users/collaborators. Nested pages, information, and links can be added as desired. Any member of the Vanderbilt community (with a valid VUNet ID and password) can create a ROCKET workspace. External users can be added to a workspace by the workspace owner/admin.
VUMC BOX
Box is an online file-sharing, cloud storage, and collaboration solution. VUMC IT provides the enterprise version of Box that allows users unlimited storage, secure access, and the ability to share files from anywhere, from any device - computers, tablets, or smartphones. VUMC use of Box requires VUNet/VUMC ID + Multi Factor Authentication (MFA). Sharing settings allow nuanced access & editing rights to files/folders by other users, including collaborators outside Vanderbilt. If Box is used for research project purposes, prior IRB approval is required.